A broadly protective therapeutic antibody against influenza B virus with two mechanisms of action
- PMID: 28102191
- PMCID: PMC5253702
- DOI: 10.1038/ncomms14234
A broadly protective therapeutic antibody against influenza B virus with two mechanisms of action
Erratum in
-
Corrigendum: A broadly protective therapeutic antibody against influenza B virus with two mechanisms of action.Nat Commun. 2017 May 25;8:15779. doi: 10.1038/ncomms15779. Nat Commun. 2017. PMID: 28541305 Free PMC article.
Abstract
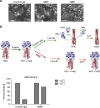
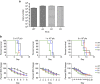
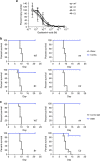
Influenza B virus (IBV) causes annual influenza epidemics around the world. Here we use an in vivo plasmablast enrichment technique to isolate a human monoclonal antibody, 46B8 that neutralizes all IBVs tested in vitro and protects mice against lethal challenge of all IBVs tested when administered 72 h post infection. 46B8 demonstrates a superior therapeutic benefit over Tamiflu and has an additive antiviral effect in combination with Tamiflu. 46B8 binds to a conserved epitope in the vestigial esterase domain of hemagglutinin (HA) and blocks HA-mediated membrane fusion. After passage of the B/Brisbane/60/2008 virus in the presence of 46B8, we isolated three resistant clones, all harbouring the same mutation (Ser301Phe) in HA that abolishes 46B8 binding to HA at low pH. Interestingly, 46B8 is still able to protect mice against lethal challenge of the mutant viruses, possibly owing to its ability to mediate antibody-dependent cellular cytotoxicity (ADCC).
Conflict of interest statement
N.C., S.P., G.N., N.C., A.E., R.F., L.K., E.K., M.R., Z.L., H.C., E.S., Z.M., J.S., M.X., P.L., C.C. and M.-W.T. are employees of Genentech; L.R.S. is an employee of Achaogen.
Figures






Similar articles
-
A multimechanistic antibody targeting the receptor binding site potently cross-protects against influenza B viruses.Sci Transl Med. 2017 Oct 18;9(412):eaam5752. doi: 10.1126/scitranslmed.aam5752. Sci Transl Med. 2017. PMID: 29046433
-
Broadly Cross-Reactive, Nonneutralizing Antibodies against Influenza B Virus Hemagglutinin Demonstrate Effector Function-Dependent Protection against Lethal Viral Challenge in Mice.J Virol. 2019 Mar 5;93(6):e01696-18. doi: 10.1128/JVI.01696-18. Print 2019 Mar 15. J Virol. 2019. PMID: 30626682 Free PMC article.
-
An IgM antibody targeting the receptor binding site of influenza B blocks viral infection with great breadth and potency.Theranostics. 2019 Jan 1;9(1):210-231. doi: 10.7150/thno.28434. eCollection 2019. Theranostics. 2019. PMID: 30662563 Free PMC article.
-
Tackling influenza with broadly neutralizing antibodies.Curr Opin Virol. 2017 Jun;24:60-69. doi: 10.1016/j.coviro.2017.03.002. Epub 2017 May 18. Curr Opin Virol. 2017. PMID: 28527859 Free PMC article. Review.
-
Analysis of the conserved protective epitopes of hemagglutinin on influenza A viruses.Front Immunol. 2023 Feb 17;14:1086297. doi: 10.3389/fimmu.2023.1086297. eCollection 2023. Front Immunol. 2023. PMID: 36875062 Free PMC article. Review.
Cited by
-
A Potent Germline-like Human Monoclonal Antibody Targets a pH-Sensitive Epitope on H7N9 Influenza Hemagglutinin.Cell Host Microbe. 2017 Oct 11;22(4):471-483.e5. doi: 10.1016/j.chom.2017.08.011. Epub 2017 Sep 28. Cell Host Microbe. 2017. PMID: 28966056 Free PMC article.
-
Monocytes Latently Infected with Human Cytomegalovirus Evade Neutrophil Killing.iScience. 2019 Feb 22;12:13-26. doi: 10.1016/j.isci.2019.01.007. Epub 2019 Jan 8. iScience. 2019. PMID: 30677738 Free PMC article.
-
Monoclonal Antibody Therapy Protects Pharmacologically Immunosuppressed Mice from Lethal Infection with Influenza B Virus.Antimicrob Agents Chemother. 2020 Aug 20;64(9):e00284-20. doi: 10.1128/AAC.00284-20. Print 2020 Aug 20. Antimicrob Agents Chemother. 2020. PMID: 32631823 Free PMC article.
-
Development of Influenza B Universal Vaccine Candidates Using the "Mosaic" Hemagglutinin Approach.J Virol. 2019 May 29;93(12):e00333-19. doi: 10.1128/JVI.00333-19. Print 2019 Jun 15. J Virol. 2019. PMID: 30944178 Free PMC article.
-
Influenza A Virus Antibodies with Antibody-Dependent Cellular Cytotoxicity Function.Viruses. 2020 Mar 1;12(3):276. doi: 10.3390/v12030276. Viruses. 2020. PMID: 32121563 Free PMC article. Review.
References
-
- Lambert L. C. & Fauci A. S. Influenza vaccines for the future. N. Engl. J. Med. 363, 2036–2044 (2010). - PubMed
-
- Yamashita M., Krystal M., Fitch W. M. & Palese P. Influenza B virus evolution: co-circulating lineages and comparison of evolutionary pattern with those of influenza A and C viruses. Virology 163, 112–122 (1988). - PubMed
-
- Centers for Disease Control and Prevention. Influenza-associated pediatric deaths--United States, September 2010-August 2011. Morb. Mortal Wkly. Rep. 60, 1233–1238 (2011). - PubMed
-
- Kawai N. et al.. A comparison of the effectiveness of oseltamivir for the treatment of influenza A and influenza B: a Japanese multicenter study of the 2003-2004 and 2004-2005 influenza seasons. Clin. Infect. Dis. 43, 439–444 (2006). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

