Whole-brain 3D mapping of human neural transplant innervation
- PMID: 28102196
- PMCID: PMC5253698
- DOI: 10.1038/ncomms14162
Whole-brain 3D mapping of human neural transplant innervation
Abstract
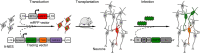
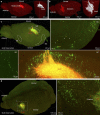
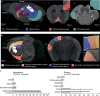
While transplantation represents a key tool for assessing in vivo functionality of neural stem cells and their suitability for neural repair, little is known about the integration of grafted neurons into the host brain circuitry. Rabies virus-based retrograde tracing has developed into a powerful approach for visualizing synaptically connected neurons. Here, we combine this technique with light sheet fluorescence microscopy (LSFM) to visualize transplanted cells and connected host neurons in whole-mouse brain preparations. Combined with co-registration of high-precision three-dimensional magnetic resonance imaging (3D MRI) reference data sets, this approach enables precise anatomical allocation of the host input neurons. Our data show that the same neural donor cell population grafted into different brain regions receives highly orthotopic input. These findings indicate that transplant connectivity is largely dictated by the circuitry of the target region and depict rabies-based transsynaptic tracing and LSFM as efficient tools for comprehensive assessment of host-donor cell innervation.
Conflict of interest statement
O.B. is a co-founder and owns equity of LIFE & BRAIN GmbH. The remaining authors declare no competing financial interests.
Figures
References
-
- Thompson L. H. & Björklund A. Reconstruction of brain circuitry by neural transplants generated from pluripotent stem cells. Neurobiol. Dis. 79, 28–40 (2015). - PubMed
-
- Debanne D. et al. Paired-recordings from synaptically coupled cortical and hippocampal neurons in acute and cultured brain slices. Nat. Protoc. 3, 1559–1568 (2008). - PubMed
-
- Svoboda K., Denk W., Kleinfeld D. & Tank D. W. In vivo dendritic calcium dynamics in neocortical pyramidal neurons. Nature 385, 161–165 (1997). - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

