Direct PIP2 binding mediates stable oligomer formation of the serotonin transporter
- PMID: 28102201
- PMCID: PMC5253637
- DOI: 10.1038/ncomms14089
Direct PIP2 binding mediates stable oligomer formation of the serotonin transporter
Abstract
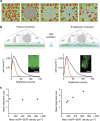
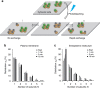
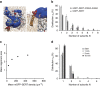
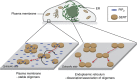
The human serotonin transporter (hSERT) mediates uptake of serotonin from the synaptic cleft and thereby terminates serotonergic signalling. We have previously found by single-molecule microscopy that SERT forms stable higher-order oligomers of differing stoichiometry at the plasma membrane of living cells. Here, we report that SERT oligomer assembly at the endoplasmic reticulum (ER) membrane follows a dynamic equilibration process, characterized by rapid exchange of subunits between different oligomers, and by a concentration dependence of the degree of oligomerization. After trafficking to the plasma membrane, however, the SERT stoichiometry is fixed. Stabilization of the oligomeric SERT complexes is mediated by the direct binding to phosphoinositide phosphatidylinositol-4,5-biphosphate (PIP2). The observed spatial decoupling of oligomer formation from the site of oligomer operation provides cells with the ability to define protein quaternary structures independent of protein density at the cell surface.
Figures

References
-
- Schloss P. & Williams D. C. The serotonin transporter: a primary target for antidepressant drugs. J. Psychopharmacol. 12, 115–121 (1998). - PubMed
-
- Kristensen A. S. et al. SLC6 neurotransmitter transporters: structure, function, and regulation. Pharmacol. Rev. 63, 585–640 (2011). - PubMed
-
- Schmid J. A. et al. Oligomerization of the human serotonin transporter and of the rat GABA transporter 1 visualized by fluorescence resonance energy transfer microscopy in living cells. J. Biol. Chem. 276, 3805–3810 (2001). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

