Chronic lymphocytic leukaemia
- PMID: 28102226
- PMCID: PMC5336551
- DOI: 10.1038/nrdp.2016.96
Chronic lymphocytic leukaemia
Abstract
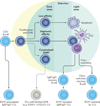
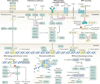
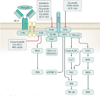
Chronic lymphocytic leukaemia (CLL) is a malignancy of CD5+ B cells that is characterized by the accumulation of small, mature-appearing lymphocytes in the blood, marrow and lymphoid tissues. Signalling via surface immunoglobulin, which constitutes the major part of the B cell receptor, and several genetic alterations play a part in CLL pathogenesis, in addition to interactions between CLL cells and other cell types, such as stromal cells, T cells and nurse-like cells in the lymph nodes. The clinical progression of CLL is heterogeneous and ranges from patients who require treatment soon after diagnosis to others who do not require therapy for many years, if at all. Several factors, including the immunoglobulin heavy-chain variable region gene (IGHV) mutational status, genomic changes, patient age and the presence of comorbidities, should be considered when defining the optimal management strategies, which include chemotherapy, chemoimmunotherapy and/or drugs targeting B cell receptor signalling or inhibitors of apoptosis, such as BCL-2. Research on the biology of CLL has profoundly enhanced our ability to identify patients who are at higher risk for disease progression and our capacity to treat patients with drugs that selectively target distinctive phenotypic or physiological features of CLL. How these and other advances have shaped our current understanding and treatment of patients with CLL is the subject of this Primer.
Figures


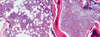

Comment in
-
Chronic lymphocytic leukaemia.Nat Rev Dis Primers. 2017 Feb 9;3:17008. doi: 10.1038/nrdp.2017.8. Nat Rev Dis Primers. 2017. PMID: 28179635 No abstract available.
References
-
- Hamblin TJ, Davis Z, Gardiner A, Oscier DG, Stevenson FK. Unmutated Ig V(H) genes are associated with a more aggressive form of chronic lymphocytic leukemia. Blood. 1999;94:1848–1854. - PubMed
-
-
Damle RN, et al. Ig V gene mutation status and CD38 expression as novel prognostic indicators in chronic lymphocytic leukemia. Blood. 1999;94:1840–1847. References and are landmark papers that describe two main subsets of patients with different disease progression tendencies based on IGHV mutation status of the immunoglobulins that are expressed by CLL cells.
-
-
- Tobin G, et al. Somatically mutated Ig V(H)3–21 genes characterize a new subset of chronic lymphocytic leukemia. Blood. 2002;99:2262–2264. - PubMed
-
-
Kipps TJ, et al. Developmentally restricted immunoglobulin heavy chain variable region gene expressed at high frequency in chronic lymphocytic leukemia. Proc. Natl Acad. Sci. USA. 1989;86:5913–5917. This paper describes the discovery that the immunoglobulin repertoire of CLL cells may be highly restricted, suggesting that the antibodies expressed by CLL cells are most likely selected based on their capacity to bind to some common self-antigens.
-
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

