The Neurosteroidogenic Enzyme 5α-Reductase Mediates Psychotic-Like Complications of Sleep Deprivation
- PMID: 28102229
- PMCID: PMC5603808
- DOI: 10.1038/npp.2017.13
The Neurosteroidogenic Enzyme 5α-Reductase Mediates Psychotic-Like Complications of Sleep Deprivation
Abstract
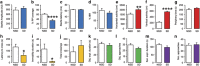
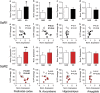
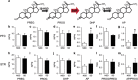
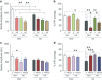
Acute sleep deprivation (SD) can trigger or exacerbate psychosis- and mania-related symptoms; the neurobiological basis of these complications, however, remains elusive. Given the extensive involvement of neuroactive steroids in psychopathology, we hypothesized that the behavioral complications of SD may be contributed by 5α-reductase (5αR), the rate-limiting enzyme in the conversion of progesterone into the neurosteroid allopregnanolone. We first tested whether rats exposed to SD may exhibit brain-regional alterations in 5αR isoenzymes and neuroactive steroid levels; then, we assessed whether the behavioral and neuroendocrine alterations induced by SD may be differentially modulated by the administration of the 5αR inhibitor finasteride, as well as progesterone and allopregnanolone. SD selectively enhanced 5αR expression and activity, as well as AP levels, in the prefrontal cortex; furthermore, finasteride (10-100 mg/kg, IP) dose-dependently ameliorated PPI deficits, hyperactivity, and risk-taking behaviors, in a fashion akin to the antipsychotic haloperidol and the mood stabilizer lithium carbonate. Finally, PPI deficits were exacerbated by allopregnanolone (10 mg/kg, IP) and attenuated by progesterone (30 mg/kg, IP) in SD-subjected, but not control rats. Collectively, these results provide the first-ever evidence that 5αR mediates a number of psychosis- and mania-like complications of SD through imbalances in cortical levels of neuroactive steroids.
Figures

References
-
- Acosta-Peña E, Camacho-Abrego I, Melgarejo-Gutiérrez M, Flores G, Drucker-Colín R, García-García F (2015). Sleep deprivation induces differential morphological changes in the hippocampus and prefrontal cortex in young and old rats. Synapse 69: 15–25. - PubMed
-
- Bauer M, Grof P, Rasgon N, Bschor T, Glenn T, Whybrow PC (2006). Temporal relation between sleep and mood in patients with bipolar disorder. Bipolar Disord 8: 160–167. - PubMed
-
- Belelli D, Lambert JJ (2005). Neurosteroids: endogenous regulators of the GABA(A)receptor. Nat Rev Neurosci 6: 565–575. - PubMed
-
- Benca RM, Obermeyer WH, Thisted RA, Gillin JC (1992). Sleep and psychiatric disorders. A meta-analysis. Arch Gen Psychiatry 49: 651–668. - PubMed
-
- Bitsios P, Giakoumaki SG (2005). Relationship of prepulse inhibition of the startle reflex to attentional and executive mechanisms in man. Int J Psychophysiol 55: 229–241. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

