Fibronectin-guided migration of carcinoma collectives
- PMID: 28102238
- PMCID: PMC5253696
- DOI: 10.1038/ncomms14105
Fibronectin-guided migration of carcinoma collectives
Abstract
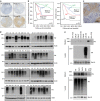
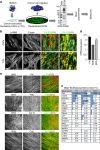
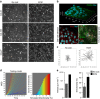
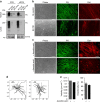
Functional interplay between tumour cells and their neoplastic extracellular matrix plays a decisive role in malignant progression of carcinomas. Here we provide a comprehensive data set of the human HNSCC-associated fibroblast matrisome. Although much attention has been paid to the deposit of collagen, we identify oncofetal fibronectin (FN) as a major and obligate component of the matrix assembled by stromal fibroblasts from head and neck squamous cell carcinomas (HNSCC). FN overexpression in tumours from 435 patients corresponds to an independent unfavourable prognostic indicator. We show that migration of carcinoma collectives on fibrillar FN-rich matrices is achieved through αvβ6 and α9β1 engagement, rather than α5β1. Moreover, αvβ6-driven migration occurs independently of latent TGF-β activation and Smad-dependent signalling in tumour epithelial cells. These results provide insights into the adhesion-dependent events at the tumour-stroma interface that govern the collective mode of migration adopted by carcinoma cells to invade surrounding stroma in HNSCC.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

