Dynamic modeling of uteroplacental blood flow in IUGR indicates vortices and elevated pressure in the intervillous space - a pilot study
- PMID: 28102332
- PMCID: PMC5244422
- DOI: 10.1038/srep40771
Dynamic modeling of uteroplacental blood flow in IUGR indicates vortices and elevated pressure in the intervillous space - a pilot study
Abstract
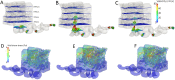
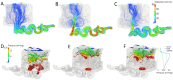
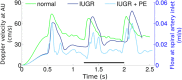
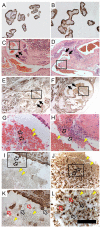
Ischemic placental disease is a concept that links intrauterine growth retardation (IUGR) and preeclampsia (PE) back to insufficient remodeling of uterine spiral arteries. The rheological consequences of insufficient remodeling of uterine spiral arteries were hypothesized to mediate the considerably later manifestation of obstetric disease. However, the micro-rheology in the intervillous space (IVS) cannot be examined clinically and rheological animal models of the human IVS do not exist. Thus, an in silico approach was implemented to provide in vivo inaccessible data. The morphology of a spiral artery and the inflow region of the IVS were three-dimensionally reconstructed to provide a morphological stage for the simulations. Advanced high-end supercomputing resources were used to provide blood flow simulations at high spatial resolution. Our simulations revealed turbulent blood flow (high-velocity jets and vortices) combined with elevated blood pressure in the IVS and increased wall shear stress at the villous surface in conjunction with insufficient spiral artery remodeling only. Post-hoc histological analysis of uterine veins showed evidence of increased trophoblast shedding in an IUGR placenta. Our data support that rheological alteration in the IVS is a relevant mechanism linking ischemic placental disease to altered structural integrity and function of the placenta.
Figures
References
-
- Ananth C. V. Ischemic placental disease: A unifying concept for preeclampsia, intrauterine growth restriction, and placental abruption. Ischemic placental disease. Semin Perinatol. 38, 131–132 (2014). - PubMed
-
- Ananth C. V. & Friedman A. M. Ischemic placental disease and risks of perinatal mortality and morbidity and neurodevelopmental outcomes. Semin Perinatol. 38, 151–158 (2014). - PubMed
-
- Rath W. Geburtshilfe und Perinatologie. 2nd ed. (Thieme, Stuttgart, 2010).
-
- Chaddha V., Viero S., Huppertz B. & Kingdom J. Developmental biology of the placenta and the origin of placental insufficiency. Semin Fetal Neonatal Med. 9, 57–69 (2004). - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

