Neural activity in the dorsal medial superior temporal area of monkeys represents retinal error during adaptive motor learning
- PMID: 28102342
- PMCID: PMC5244411
- DOI: 10.1038/srep40939
Neural activity in the dorsal medial superior temporal area of monkeys represents retinal error during adaptive motor learning
Abstract
To adapt to variable environments, humans regulate their behavior by modulating gains in sensory-to-motor processing. In this study, we measured a simple eye movement, the ocular following response (OFR), in monkeys to study the neuronal basis of adaptive motor learning in the visuomotor processing stream. The medial superior temporal (MST) area of the cerebral cortex is a critical site for contextual gain modulation of the OFR. However, the role of MST neurons in adaptive gain modulation of the OFR remains unknown. We adopted a velocity step-down sequence paradigm that was designed to promote adaptive gain modulation of the OFR to investigate the role of the dorsal MST (MSTd) in adaptive motor learning. In the initial learning stage, we observed a reduction in the OFR but no significant change in the "open-loop" responses for the majority of the MSTd neurons. However, in the late learning stage, some MSTd neurons exhibited significantly enhanced "closed-loop" responses in association with increases in retinal error velocity. These results indicate that the MSTd area primarily encodes visual motion, suggesting that MSTd neurons function upstream of the motor learning site to provide sensory signals to the downstream structures involved in adaptive motor learning.
Figures




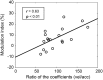
References
-
- Miles F. A., Kawano K. & Optican L. M. Short-latency ocular following responses of monkey. I. Dependence on temporospatial properties of visual input. Journal of neurophysiology 56, 1321–1354 (1986). - PubMed
-
- Gellman R. S., Carl J. R. & Miles F. A. Short latency ocular-following responses in man. Visual neuroscience 5, 107–122 (1990). - PubMed
-
- Schwarz U. & Miles F. A. Ocular responses to translation and their dependence on viewing distance. I. Motion of the observer. Journal of neurophysiology 66, 851–864 (1991). - PubMed
-
- Busettini C., Miles F. A. & Schwarz U. Ocular responses to translation and their dependence on viewing distance. II. Motion of the scene. Journal of neurophysiology 66, 865–878 (1991). - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

