Interspecies cathelicidin comparison reveals divergence in antimicrobial activity, TLR modulation, chemokine induction and regulation of phagocytosis
- PMID: 28102367
- PMCID: PMC5244392
- DOI: 10.1038/srep40874
Interspecies cathelicidin comparison reveals divergence in antimicrobial activity, TLR modulation, chemokine induction and regulation of phagocytosis
Abstract
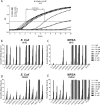
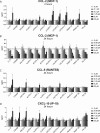
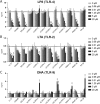
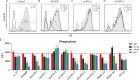
Cathelicidins are short cationic peptides initially described as antimicrobial peptides, which can also modulate the immune system. Because most findings have been described in the context of human LL-37 or murine CRAMP, or have been investigated under varying conditions, it is unclear which functions are cathelicidin specific and which functions are general cathelicidin properties. This study compares 12 cathelicidins from 6 species under standardized conditions to better understand the conservation of cathelicidin functions. Most tested cathelicidins had strong antimicrobial activity against E. coli and/or MRSA. Interestingly, while more physiological culture conditions limit the antimicrobial activity of almost all cathelicidins against E. coli, activity against MRSA is enhanced. Seven out of 12 cathelicidins were able to neutralize LPS and another 7 cathelicidins were able to neutralize LTA; however, there was no correlation found with LPS neutralization. In contrast, only 4 cathelicidins enhanced DNA-induced TLR9 activation. In conclusion, these results provide new insight in the functional differences of cathelicidins both within and between species. In addition, these results underline the importance not to generalize cathelicidin functions and indicates that caution should be taken in extrapolating results from LL-37- or CRAMP-related studies to other animal settings.
Figures
References
-
- Zasloff M. Antimicrobial peptides of multicellular organisms. Nature 415, 389–395 (2002). - PubMed
-
- Gudmundsson G. H. et al.. The human gene FALL39 and processing of the cathelin precursor to the antibacterial peptide LL-37 in granulocytes. Eur. J. Biochem. 238, 325–332 (1996). - PubMed
-
- Agerberth B. et al.. The human antimicrobial and chemotactic peptides LL-37 and alpha-defensins are expressed by specific lymphocyte and monocyte populations. Blood 96, 3086–3093 (2000). - PubMed
-
- Larrick J. W. et al.. Structural, functional analysis and localization of the human CAP18 gene. FEBS Lett. 398, 74–80 (1996). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

