Single-Walled Carbon Nanotubes: Mimics of Biological Ion Channels
- PMID: 28103039
- PMCID: PMC5301282
- DOI: 10.1021/acs.nanolett.6b04967
Single-Walled Carbon Nanotubes: Mimics of Biological Ion Channels
Abstract
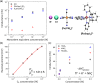
Here we report on the ion conductance through individual, small diameter single-walled carbon nanotubes. We find that they are mimics of ion channels found in natural systems. We explore the factors governing the ion selectivity and permeation through single-walled carbon nanotubes by considering an electrostatic mechanism built around a simplified version of the Gouy-Chapman theory. We find that the single-walled carbon nanotubes preferentially transported cations and that the cation permeability is size-dependent. The ionic conductance increases as the absolute hydration enthalpy decreases for monovalent cations with similar solid-state radii, hydrated radii, and bulk mobility. Charge screening experiments using either the addition of cationic or anionic polymers, divalent metal cations, or changes in pH reveal the enormous impact of the negatively charged carboxylates at the entrance of the single-walled carbon nanotubes. These observations were modeled in the low-to-medium concentration range (0.1-2.0 M) by an electrostatic mechanism that mimics the behavior observed in many biological ion channel-forming proteins. Moreover, multi-ion conduction in the high concentration range (>2.0 M) further reinforces the similarity between single-walled carbon nanotubes and protein ion channels.
Keywords: Ion channel; multi-ion conduction; nanofluidic device; single-walled carbon nanotubes.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



Similar articles
-
Ion exclusion by sub-2-nm carbon nanotube pores.Proc Natl Acad Sci U S A. 2008 Nov 11;105(45):17250-5. doi: 10.1073/pnas.0710437105. Epub 2008 Jun 6. Proc Natl Acad Sci U S A. 2008. PMID: 18539773 Free PMC article.
-
Synthetic cation-selective nanotube: permeant cations chaperoned by anions.J Chem Phys. 2011 Jan 28;134(4):045103. doi: 10.1063/1.3524310. J Chem Phys. 2011. PMID: 21280804
-
pH-tunable ion selectivity in carbon nanotube pores.Langmuir. 2010 Sep 21;26(18):14848-53. doi: 10.1021/la101943h. Langmuir. 2010. PMID: 20715879
-
Single-walled carbon nanotubes as near-infrared optical biosensors for life sciences and biomedicine.Biotechnol J. 2015 Mar;10(3):447-59. doi: 10.1002/biot.201400168. Epub 2015 Feb 13. Biotechnol J. 2015. PMID: 25676253 Review.
-
Computational modeling of transport in synthetic nanotubes.Nanomedicine. 2011 Dec;7(6):702-9. doi: 10.1016/j.nano.2011.02.011. Epub 2011 Mar 17. Nanomedicine. 2011. PMID: 21419868 Review.
Cited by
-
Comediation of voltage gating and ion charge in MXene membrane for controllable and selective monovalent cation separation.Sci Adv. 2024 Dec 6;10(49):eado3998. doi: 10.1126/sciadv.ado3998. Epub 2024 Dec 4. Sci Adv. 2024. PMID: 39630891 Free PMC article.
-
Ions and Water Dancing through Atom-Scale Holes: A Perspective toward "Size Zero".ACS Nano. 2020 Apr 28;14(4):3736-3746. doi: 10.1021/acsnano.0c01625. Epub 2020 Mar 20. ACS Nano. 2020. PMID: 32195580 Free PMC article.
-
Bio-inspired pores for selective ion transport.Natl Sci Rev. 2025 Jan 23;12(3):nwaf025. doi: 10.1093/nsr/nwaf025. eCollection 2025 Mar. Natl Sci Rev. 2025. PMID: 39958144 Free PMC article. No abstract available.
-
Carbon nanoparticles enhance potassium uptake via upregulating potassium channel expression and imitating biological ion channels in BY-2 cells.J Nanobiotechnology. 2020 Jan 28;18(1):21. doi: 10.1186/s12951-020-0581-0. J Nanobiotechnology. 2020. PMID: 31992314 Free PMC article.
-
Influence of effective polarization on ion and water interactions within a biomimetic nanopore.Biophys J. 2022 Jun 7;121(11):2014-2026. doi: 10.1016/j.bpj.2022.05.006. Epub 2022 May 7. Biophys J. 2022. PMID: 35527400 Free PMC article.
References
-
- Hille B.Ion Channels of Excitable Membranes, 3rd ed.; Sinauer: Sunderland, MA, 2001; p xviii.
-
- Dworakowska B.; Dolowy K. Acta Biochim. Polym. 2000, 47 (3), 685–703. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

