Bombyx mori P-element Somatic Inhibitor (BmPSI) Is a Key Auxiliary Factor for Silkworm Male Sex Determination
- PMID: 28103247
- PMCID: PMC5289617
- DOI: 10.1371/journal.pgen.1006576
Bombyx mori P-element Somatic Inhibitor (BmPSI) Is a Key Auxiliary Factor for Silkworm Male Sex Determination
Abstract
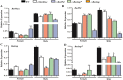
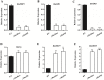
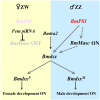
Manipulation of sex determination pathways in insects provides the basis for a wide spectrum of strategies to benefit agriculture and public health. Furthermore, insects display a remarkable diversity in the genetic pathways that lead to sex differentiation. The silkworm, Bombyx mori, has been cultivated by humans as a beneficial insect for over two millennia, and more recently as a model system for studying lepidopteran genetics and development. Previous studies have identified the B. mori Fem piRNA as the primary female determining factor and BmMasc as its downstream target, while the genetic scenario for male sex determination was still unclear. In the current study, we exploite the transgenic CRISPR/Cas9 system to generate a comprehensive set of knockout mutations in genes BmSxl, Bmtra2, BmImp, BmImpM, BmPSI and BmMasc, to investigate their roles in silkworm sex determination. Absence of Bmtra2 results in the complete depletion of Bmdsx transcripts, which is the conserved downstream factor in the sex determination pathway, and induces embryonic lethality. Loss of BmImp or BmImpM function does not affect the sexual differentiation. Mutations in BmPSI and BmMasc genes affect the splicing of Bmdsx and the female reproductive apparatus appeared in the male external genital. Intriguingly, we identify that BmPSI regulates expression of BmMasc, BmImpM and Bmdsx, supporting the conclusion that it acts as a key auxiliary factor in silkworm male sex determination.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




Similar articles
-
The Masc-PSI complex directly induces male-type doublesex splicing in silkworms.Commun Biol. 2025 Jun 14;8(1):927. doi: 10.1038/s42003-025-08350-y. Commun Biol. 2025. PMID: 40517142 Free PMC article.
-
MicroRNA-2738 regulates gene expression in the sex determination pathway in Bombyx mori.Insect Sci. 2020 Aug;27(4):646-654. doi: 10.1111/1744-7917.12694. Epub 2019 Jun 17. Insect Sci. 2020. PMID: 31131541
-
Analysis of interaction between Bmhrp28 and BmPSI in sex-specific splicing of Bombyx mori Bmdsx gene.Genet Mol Res. 2014 Jul 24;13(3):5452-62. doi: 10.4238/2014.July.24.25. Genet Mol Res. 2014. PMID: 25078602
-
Sex determination in the silkworm, Bombyx mori: a female determinant on the W chromosome and the sex-determining gene cascade.Semin Cell Dev Biol. 2007 Jun;18(3):379-88. doi: 10.1016/j.semcdb.2007.02.008. Epub 2007 Mar 1. Semin Cell Dev Biol. 2007. PMID: 17446095 Review.
-
The Sex Determination Cascade in the Silkworm.Genes (Basel). 2021 Feb 23;12(2):315. doi: 10.3390/genes12020315. Genes (Basel). 2021. PMID: 33672402 Free PMC article. Review.
Cited by
-
High-throughput and genome-scale targeted mutagenesis using CRISPR in a nonmodel multicellular organism, Bombyx mori.Genome Res. 2024 Feb 7;34(1):134-144. doi: 10.1101/gr.278297.123. Genome Res. 2024. PMID: 38191205 Free PMC article.
-
The Masc-PSI complex directly induces male-type doublesex splicing in silkworms.Commun Biol. 2025 Jun 14;8(1):927. doi: 10.1038/s42003-025-08350-y. Commun Biol. 2025. PMID: 40517142 Free PMC article.
-
A determining factor for insect feeding preference in the silkworm, Bombyx mori.PLoS Biol. 2019 Feb 27;17(2):e3000162. doi: 10.1371/journal.pbio.3000162. eCollection 2019 Feb. PLoS Biol. 2019. PMID: 30811402 Free PMC article.
-
CRISPR disruption of TCTP gene impaired normal development in the silkworm Bombyx mori.Insect Sci. 2019 Dec;26(6):973-982. doi: 10.1111/1744-7917.12567. Epub 2018 Feb 19. Insect Sci. 2019. PMID: 29316276 Free PMC article.
-
Molecular Disruption of Ion Transport Peptide Receptor Results in Impaired Water Homeostasis and Developmental Defects in Bombyx mori.Front Physiol. 2020 May 20;11:424. doi: 10.3389/fphys.2020.00424. eCollection 2020. Front Physiol. 2020. PMID: 32508668 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

