Effect of the Latent Reservoir on the Evolution of HIV at the Within- and Between-Host Levels
- PMID: 28103248
- PMCID: PMC5245781
- DOI: 10.1371/journal.pcbi.1005228
Effect of the Latent Reservoir on the Evolution of HIV at the Within- and Between-Host Levels
Abstract
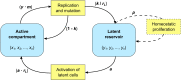
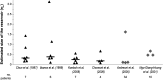
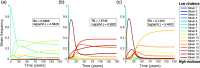
The existence of long-lived reservoirs of latently infected CD4+ T cells is the major barrier to curing HIV, and has been extensively studied in this light. However, the effect of these reservoirs on the evolutionary dynamics of the virus has received little attention. Here, we present a within-host quasispecies model that incorporates a long-lived reservoir, which we then nest into an epidemiological model of HIV dynamics. For biologically plausible parameter values, we find that the presence of a latent reservoir can severely delay evolutionary dynamics within a single host, with longer delays associated with larger relative reservoir sizes and/or homeostatic proliferation of cells within the reservoir. These delays can fundamentally change the dynamics of the virus at the epidemiological scale. In particular, the delay in within-host evolutionary dynamics can be sufficient for the virus to evolve intermediate viral loads consistent with maximising transmission, as is observed, and not the very high viral loads that previous models have predicted, an effect that can be further enhanced if viruses similar to those that initiate infection are preferentially transmitted. These results depend strongly on within-host characteristics such as the relative reservoir size, with the evolution of intermediate viral loads observed only when the within-host dynamics are sufficiently delayed. In conclusion, we argue that the latent reservoir has important, and hitherto under-appreciated, roles in both within- and between-host viral evolution.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


Similar articles
-
Longitudinal clonal dynamics of HIV-1 latent reservoirs measured by combination quadruplex polymerase chain reaction and sequencing.Proc Natl Acad Sci U S A. 2022 Jan 25;119(4):e2117630119. doi: 10.1073/pnas.2117630119. Proc Natl Acad Sci U S A. 2022. PMID: 35042816 Free PMC article.
-
Stable Phenotypic Changes of the Host T Cells Are Essential to the Long-Term Stability of Latent HIV-1 Infection.J Virol. 2015 Jul;89(13):6656-72. doi: 10.1128/JVI.00571-15. Epub 2015 Apr 15. J Virol. 2015. PMID: 25878110 Free PMC article.
-
Myeloid and CD4 T Cells Comprise the Latent Reservoir in Antiretroviral Therapy-Suppressed SIVmac251-Infected Macaques.mBio. 2019 Aug 20;10(4):e01659-19. doi: 10.1128/mBio.01659-19. mBio. 2019. PMID: 31431552 Free PMC article.
-
HIV latency.Cold Spring Harb Perspect Med. 2011 Sep;1(1):a007096. doi: 10.1101/cshperspect.a007096. Cold Spring Harb Perspect Med. 2011. PMID: 22229121 Free PMC article. Review.
-
The challenge of viral reservoirs in HIV-1 infection.Annu Rev Med. 2002;53:557-93. doi: 10.1146/annurev.med.53.082901.104024. Annu Rev Med. 2002. PMID: 11818490 Review.
Cited by
-
Multi-scale immunoepidemiological modeling of within-host and between-host HIV dynamics: systematic review of mathematical models.PeerJ. 2017 Sep 28;5:e3877. doi: 10.7717/peerj.3877. eCollection 2017. PeerJ. 2017. PMID: 28970973 Free PMC article.
-
Machine learning aided multiscale modelling of the HIV-1 infection in the presence of NRTI therapy.PeerJ. 2023 Mar 31;11:e15033. doi: 10.7717/peerj.15033. eCollection 2023. PeerJ. 2023. PMID: 37020854 Free PMC article.
-
Short-Sighted Virus Evolution and a Germline Hypothesis for Chronic Viral Infections.Trends Microbiol. 2017 May;25(5):336-348. doi: 10.1016/j.tim.2017.03.003. Epub 2017 Apr 1. Trends Microbiol. 2017. PMID: 28377208 Free PMC article. Review.
-
Theoretical conditions for the coexistence of viral strains with differences in phenotypic traits: a bifurcation analysis.R Soc Open Sci. 2019 Jan 9;6(1):181179. doi: 10.1098/rsos.181179. eCollection 2019 Jan. R Soc Open Sci. 2019. PMID: 30800366 Free PMC article.
-
Evaluating the long-term effects of combination antiretroviral therapy of HIV infection: a modeling study.J Math Biol. 2025 Mar 1;90(4):36. doi: 10.1007/s00285-025-02196-y. J Math Biol. 2025. PMID: 40025191 Free PMC article.
References
-
- Finzi D., Hermankova M., Pierson T., Carruth L. M., Buck C., Chaisson R. E., et al. Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science. 1997; 278(5341): 1295–1300. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

