Tumor Infiltration in Enhancing and Non-Enhancing Parts of Glioblastoma: A Correlation with Histopathology
- PMID: 28103256
- PMCID: PMC5245878
- DOI: 10.1371/journal.pone.0169292
Tumor Infiltration in Enhancing and Non-Enhancing Parts of Glioblastoma: A Correlation with Histopathology
Abstract
Purpose: To correlate histopathologic findings from biopsy specimens with their corresponding location within enhancing areas, non-enhancing areas and necrotic areas on contrast enhanced T1-weighted MRI scans (cT1).
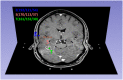
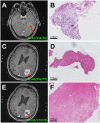
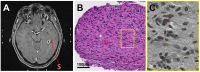
Materials and methods: In 37 patients with newly diagnosed glioblastoma who underwent stereotactic biopsy, we obtained a correlation of 561 1mm3 biopsy specimens with their corresponding position on the intraoperative cT1 image at 1.5 Tesla. Biopsy points were categorized as enhancing (CE), non-enhancing (NE) or necrotic (NEC) on cT1 and tissue samples were categorized as "viable tumor cells", "blood" or "necrotic tissue (with or without cellular component)". Cell counting was done semi-automatically.
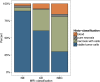
Results: NE had the highest content of tissue categorized as viable tumor cells (89% vs. 60% in CE and 30% NEC, respectively). Besides, the average cell density for NE (3764 ± 2893 cells/mm2) was comparable to CE (3506 ± 3116 cells/mm2), while NEC had a lower cell density with 2713 ± 3239 cells/mm2. If necrotic parts and bleeds were excluded, cell density in biopsies categorized as "viable tumor tissue" decreased from the center of the tumor (NEC, 5804 ± 3480 cells/mm2) to CE (4495 ± 3209 cells/mm2) and NE (4130 ± 2817 cells/mm2).
Discussion: The appearance of a glioblastoma on a cT1 image (circular enhancement, central necrosis, peritumoral edema) does not correspond to its diffuse histopathological composition. Cell density is elevated in both CE and NE parts. Hence, our study suggests that NE contains considerable amounts of infiltrative tumor with a high cellularity which might be considered in resection planning.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
-
- Sanai N, Berger MS. Glioma extent of resection and its impact on patient outcome. Neurosurgery. 2008;62(4):753–64; discussion 264–6. 10.1227/01.neu.0000318159.21731.cf - DOI - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

