Prenatal Exposure to Lipopolysaccharide Alters Renal DNA Methyltransferase Expression in Rat Offspring
- PMID: 28103274
- PMCID: PMC5245821
- DOI: 10.1371/journal.pone.0169206
Prenatal Exposure to Lipopolysaccharide Alters Renal DNA Methyltransferase Expression in Rat Offspring
Abstract
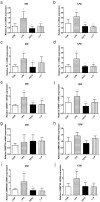
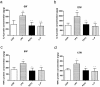
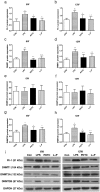
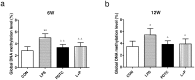
Prenatal exposure to inflammation results in hypertension during adulthood but the mechanisms are not well understood. Maternal exposure to lipopolysaccharide (LPS) alters interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α) levels in the fetal environment. As reported in many recent studies, IL-6 regulates DNA methyltransferases (DNMTs) through the transcription factor friend leukemia virus integration 1 (Fli-1). The present study explores the role of intrarenal DNMTs during development of hypertension induced by prenatal exposure to LPS. Pregnant rats were randomly divided into four treatment groups: control, LPS, pyrrolidine dithiocarbamate (PDTC, a NF-κB inhibitor), and the combination of LPS and PDTC. Expression of IL-6, Fli-1, TNF-α, DNMT1 and DNMT3B was significantly increased in the offspring of LPS-treated rats. Global DNA methylation level of renal cortex also increased dramatically in rat offspring of the LPS group. Prenatal PDTC administration reversed the increases in gene expression and global DNA methylation level. These findings suggest that prenatal exposure to LPS may result in changes of intrarenal DNMTs through the IL-6/Fli-1 pathway and TNF-α, which probably involves hypertension in offspring due to maternal exposure to inflammation.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
Post-Natal Inhibition of NF-κB Activation Prevents Renal Damage Caused by Prenatal LPS Exposure.PLoS One. 2016 Apr 13;11(4):e0153434. doi: 10.1371/journal.pone.0153434. eCollection 2016. PLoS One. 2016. PMID: 27073902 Free PMC article.
-
Prenatal exposure to lipopolysaccharide alters the intrarenal renin-angiotensin system and renal damage in offspring rats.Hypertens Res. 2010 Jan;33(1):76-82. doi: 10.1038/hr.2009.185. Epub 2009 Nov 13. Hypertens Res. 2010. PMID: 19911002
-
Effect of prenatal exposure to LPS combined with pre- and post-natal high-fat diet on hippocampus in rat offspring.Neuroscience. 2015 Feb 12;286:364-70. doi: 10.1016/j.neuroscience.2014.12.002. Epub 2014 Dec 10. Neuroscience. 2015. PMID: 25498225
-
Prenatal Programming of Neuroendocrine System Development by Lipopolysaccharide: Long-Term Effects.Int J Mol Sci. 2018 Nov 21;19(11):3695. doi: 10.3390/ijms19113695. Int J Mol Sci. 2018. PMID: 30469423 Free PMC article. Review.
-
Role of Transcription Factor Fli-1 in Inflammation and Autoimmune Diseases.Biomolecules. 2025 Mar 25;15(4):480. doi: 10.3390/biom15040480. Biomolecules. 2025. PMID: 40305194 Free PMC article. Review.
Cited by
-
Fli-1 Activation through Targeted Promoter Activity Regulation Using a Novel 3', 5'-diprenylated Chalcone Inhibits Growth and Metastasis of Prostate Cancer Cells.Int J Mol Sci. 2020 Mar 23;21(6):2216. doi: 10.3390/ijms21062216. Int J Mol Sci. 2020. PMID: 32210104 Free PMC article.
-
Chromatin modifiers in human disease: from functional roles to regulatory mechanisms.Mol Biomed. 2024 Apr 8;5(1):12. doi: 10.1186/s43556-024-00175-1. Mol Biomed. 2024. PMID: 38584203 Free PMC article. Review.
-
The First Thousand Days: Kidney Health and Beyond.Healthcare (Basel). 2021 Oct 6;9(10):1332. doi: 10.3390/healthcare9101332. Healthcare (Basel). 2021. PMID: 34683012 Free PMC article. Review.
-
LPS versus Poly I:C model: comparison of long-term effects of bacterial and viral maternal immune activation on the offspring.Am J Physiol Regul Integr Comp Physiol. 2022 Feb 1;322(2):R99-R111. doi: 10.1152/ajpregu.00087.2021. Epub 2021 Dec 7. Am J Physiol Regul Integr Comp Physiol. 2022. PMID: 34874190 Free PMC article. Review.
-
Preventive Aspects of Early Resveratrol Supplementation in Cardiovascular and Kidney Disease of Developmental Origins.Int J Mol Sci. 2021 Apr 19;22(8):4210. doi: 10.3390/ijms22084210. Int J Mol Sci. 2021. PMID: 33921641 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

