Mycobacterium marinum Degrades Both Triacylglycerols and Phospholipids from Its Dictyostelium Host to Synthesise Its Own Triacylglycerols and Generate Lipid Inclusions
- PMID: 28103313
- PMCID: PMC5245797
- DOI: 10.1371/journal.ppat.1006095
Mycobacterium marinum Degrades Both Triacylglycerols and Phospholipids from Its Dictyostelium Host to Synthesise Its Own Triacylglycerols and Generate Lipid Inclusions
Abstract
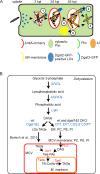
During a tuberculosis infection and inside lipid-laden foamy macrophages, fatty acids (FAs) and sterols are the major energy and carbon source for Mycobacterium tuberculosis. Mycobacteria can be found both inside a vacuole and the cytosol, but how this impacts their access to lipids is not well appreciated. Lipid droplets (LDs) store FAs in form of triacylglycerols (TAGs) and are energy reservoirs of prokaryotes and eukaryotes. Using the Dictyostelium discoideum/Mycobacterium marinum infection model we showed that M. marinum accesses host LDs to build up its own intracytosolic lipid inclusions (ILIs). Here, we show that host LDs aggregate at regions of the bacteria that become exposed to the cytosol, and appear to coalesce on their hydrophobic surface leading to a transfer of diacylglycerol O-acyltransferase 2 (Dgat2)-GFP onto the bacteria. Dictyostelium knockout mutants for both Dgat enzymes are unable to generate LDs. Instead, the excess of exogenous FAs is esterified predominantly into phospholipids, inducing uncontrolled proliferation of the endoplasmic reticulum (ER). Strikingly, in absence of host LDs, M. marinum alternatively exploits these phospholipids, resulting in rapid reversal of ER-proliferation. In addition, the bacteria are unable to restrict their acquisition of lipids from the dgat1&2 double knockout leading to vast accumulation of ILIs. Recent data indicate that the presence of ILIs is one of the characteristics of dormant mycobacteria. During Dictyostelium infection, ILI formation in M. marinum is not accompanied by a significant change in intracellular growth and a reduction in metabolic activity, thus providing evidence that storage of neutral lipids does not necessarily induce dormancy.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures






Similar articles
-
Lipid droplet dynamics at early stages of Mycobacterium marinum infection in Dictyostelium.Cell Microbiol. 2015 Sep;17(9):1332-49. doi: 10.1111/cmi.12437. Epub 2015 Apr 8. Cell Microbiol. 2015. PMID: 25772333
-
Breaking fat! How mycobacteria and other intracellular pathogens manipulate host lipid droplets.Biochimie. 2017 Oct;141:54-61. doi: 10.1016/j.biochi.2017.06.001. Epub 2017 Jun 3. Biochimie. 2017. PMID: 28587792 Review.
-
Zn2+ Intoxication of Mycobacterium marinum during Dictyostelium discoideum Infection Is Counteracted by Induction of the Pathogen Zn2+ Exporter CtpC.mBio. 2021 Feb 2;12(1):e01313-20. doi: 10.1128/mBio.01313-20. mBio. 2021. PMID: 33531393 Free PMC article.
-
Flotillin and RacH modulate the intracellular immunity of Dictyostelium to Mycobacterium marinum infection.Cell Microbiol. 2007 Nov;9(11):2716-33. doi: 10.1111/j.1462-5822.2007.00993.x. Epub 2007 Jun 24. Cell Microbiol. 2007. PMID: 17587329
-
Exploring host-pathogen interactions in the Dictyostelium discoideum-Mycobacterium marinum infection model of tuberculosis.Dis Model Mech. 2024 Jul 1;17(7):dmm050698. doi: 10.1242/dmm.050698. Epub 2024 Jul 22. Dis Model Mech. 2024. PMID: 39037280 Free PMC article. Review.
Cited by
-
Transcriptional Responses of Dictyostelium discoideum Exposed to Different Classes of Bacteria.Front Microbiol. 2020 Mar 10;11:410. doi: 10.3389/fmicb.2020.00410. eCollection 2020. Front Microbiol. 2020. PMID: 32210949 Free PMC article.
-
Metabolic Versatility of Mycobacterium tuberculosis during Infection and Dormancy.Metabolites. 2021 Feb 2;11(2):88. doi: 10.3390/metabo11020088. Metabolites. 2021. PMID: 33540752 Free PMC article. Review.
-
Analogues of Pyrimidine Nucleosides as Mycobacteria Growth Inhibitors.Microorganisms. 2022 Jun 27;10(7):1299. doi: 10.3390/microorganisms10071299. Microorganisms. 2022. PMID: 35889017 Free PMC article. Review.
-
Molecular Events Occurring in Lipophagy and Its Regulation in Flaviviridae Infection.Front Microbiol. 2021 May 21;12:651952. doi: 10.3389/fmicb.2021.651952. eCollection 2021. Front Microbiol. 2021. PMID: 34093468 Free PMC article. Review.
-
A glycine-rich PE_PGRS protein governs mycobacterial actin-based motility.Nat Commun. 2022 Jun 24;13(1):3608. doi: 10.1038/s41467-022-31333-0. Nat Commun. 2022. PMID: 35750685 Free PMC article.
References
-
- D'Avila H, Melo RC, Parreira GG, Werneck-Barroso E, Castro-Faria-Neto HC, Bozza PT. Mycobacterium bovis bacillus Calmette-Guerin induces TLR2-mediated formation of lipid bodies: intracellular domains for eicosanoid synthesis in vivo. J Immunol. 2006;176: 3087–97. - PubMed
-
- de Mattos KA, Sarno EN, Pessolani MC, Bozza PT. Deciphering the contribution of lipid droplets in leprosy: multifunctional organelles with roles in Mycobacterium leprae pathogenesis. Mem Inst Oswaldo Cruz. 2012;107 Suppl 1: 156–66. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

