HTLV-1 Tax Induces Formation of the Active Macromolecular IKK Complex by Generating Lys63- and Met1-Linked Hybrid Polyubiquitin Chains
- PMID: 28103322
- PMCID: PMC5283754
- DOI: 10.1371/journal.ppat.1006162
HTLV-1 Tax Induces Formation of the Active Macromolecular IKK Complex by Generating Lys63- and Met1-Linked Hybrid Polyubiquitin Chains
Abstract
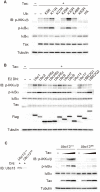
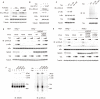
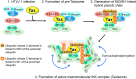
The Tax protein of human T-cell leukemia virus type 1 (HTLV-1) is crucial for the development of adult T-cell leukemia (ATL), a highly malignant CD4+ T cell neoplasm. Among the multiple aberrant Tax-induced effects on cellular processes, persistent activation of transcription factor NF-κB, which is activated only transiently upon physiological stimulation, is essential for leukemogenesis. We and others have shown that Tax induces activation of the IκB kinase (IKK) complex, which is a critical step in NF-κB activation, by generating Lys63-linked polyubiquitin chains. However, the molecular mechanism underlying Tax-induced IKK activation is controversial and not fully understood. Here, we demonstrate that Tax recruits linear (Met1-linked) ubiquitin chain assembly complex (LUBAC) to the IKK complex and that Tax fails to induce IKK activation in cells that lack LUBAC activity. Mass spectrometric analyses revealed that both Lys63-linked and Met1-linked polyubiquitin chains are associated with the IKK complex. Furthermore, treatment of the IKK-associated polyubiquitin chains with Met1-linked-chain-specific deubiquitinase (OTULIN) resulted in the reduction of high molecular weight polyubiquitin chains and the generation of short Lys63-linked ubiquitin chains, indicating that Tax can induce the generation of Lys63- and Met1-linked hybrid polyubiquitin chains. We also demonstrate that Tax induces formation of the active macromolecular IKK complex and that the blocking of Tax-induced polyubiquitin chain synthesis inhibited formation of the macromolecular complex. Taken together, these results lead us to propose a novel model in which the hybrid-chain-dependent oligomerization of the IKK complex triggered by Tax leads to trans-autophosphorylation-mediated IKK activation.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


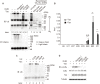

Similar articles
-
HTLV-1 Tax Functions as a Ubiquitin E3 Ligase for Direct IKK Activation via Synthesis of Mixed-Linkage Polyubiquitin Chains.PLoS Pathog. 2016 Apr 15;12(4):e1005584. doi: 10.1371/journal.ppat.1005584. eCollection 2016 Apr. PLoS Pathog. 2016. PMID: 27082114 Free PMC article.
-
HTLV-1 Tax Stimulates Ubiquitin E3 Ligase, Ring Finger Protein 8, to Assemble Lysine 63-Linked Polyubiquitin Chains for TAK1 and IKK Activation.PLoS Pathog. 2015 Aug 18;11(8):e1005102. doi: 10.1371/journal.ppat.1005102. eCollection 2015 Aug. PLoS Pathog. 2015. PMID: 26285145 Free PMC article.
-
Activation of the IκB kinase complex by HTLV-1 Tax requires cytosolic factors involved in Tax-induced polyubiquitination.J Biochem. 2011 Dec;150(6):679-86. doi: 10.1093/jb/mvr106. Epub 2011 Aug 23. J Biochem. 2011. PMID: 21862596
-
Activation of I-kappaB kinase by the HTLV type 1 Tax protein: mechanistic insights into the adaptor function of IKKgamma.AIDS Res Hum Retroviruses. 2000 Nov 1;16(16):1591-6. doi: 10.1089/08892220050193001. AIDS Res Hum Retroviruses. 2000. PMID: 11080796 Review.
-
Regulation of HTLV-1 tax stability, cellular trafficking and NF-κB activation by the ubiquitin-proteasome pathway.Viruses. 2014 Oct 23;6(10):3925-43. doi: 10.3390/v6103925. Viruses. 2014. PMID: 25341660 Free PMC article. Review.
Cited by
-
NF-κB-Induced R-Loops and Genomic Instability in HTLV-1-Infected and Adult T-Cell Leukemia Cells.Viruses. 2022 Apr 23;14(5):877. doi: 10.3390/v14050877. Viruses. 2022. PMID: 35632619 Free PMC article. Review.
-
SQSTM-1/p62 potentiates HTLV-1 Tax-mediated NF-κB activation through its ubiquitin binding function.Sci Rep. 2019 Nov 5;9(1):16014. doi: 10.1038/s41598-019-52408-x. Sci Rep. 2019. PMID: 31690813 Free PMC article.
-
Strategies of Human T-Cell Leukemia Virus Type 1 for Persistent Infection: Implications for Leukemogenesis of Adult T-Cell Leukemia-Lymphoma.Front Microbiol. 2020 May 19;11:979. doi: 10.3389/fmicb.2020.00979. eCollection 2020. Front Microbiol. 2020. PMID: 32508789 Free PMC article. Review.
-
Specificity in Ubiquitination Triggered by Virus Infection.Int J Mol Sci. 2020 Jun 8;21(11):4088. doi: 10.3390/ijms21114088. Int J Mol Sci. 2020. PMID: 32521668 Free PMC article. Review.
-
Mechanisms of Oncogenesis by HTLV-1 Tax.Pathogens. 2020 Jul 7;9(7):543. doi: 10.3390/pathogens9070543. Pathogens. 2020. PMID: 32645846 Free PMC article. Review.
References
-
- Gessain A, Mahieux R. Tropical spastic paraparesis and HTLV-1 associated myelopathy: clinical, epidemiological, virological and therapeutic aspects. Rev Neurol (Paris). 2012;168(3):257–69. Epub 2012/03/13. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

