Analytical characterization and pharmacological evaluation of the new psychoactive substance 4-fluoromethylphenidate (4F-MPH) and differentiation between the (±)-threo and (±)-erythro diastereomers
- PMID: 28103426
- PMCID: PMC5378611
- DOI: 10.1002/dta.2167
Analytical characterization and pharmacological evaluation of the new psychoactive substance 4-fluoromethylphenidate (4F-MPH) and differentiation between the (±)-threo and (±)-erythro diastereomers
Abstract
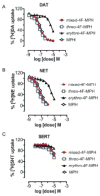
Misuse of (±)-threo-methylphenidate (methyl-2-phenyl-2-(piperidin-2-yl)acetate; Ritalin®; MPH) has long been acknowledged, but the appearance of MPH analogs in the form of 'research chemicals' has only emerged in more recent years. 4-Fluoromethylphenidate (4F-MPH) is one of these recent examples. This study presents the identification and analytical characterization of two powdered 4F-MPH products that were obtained from an online vendor in 2015. Interestingly, the products appeared to have originated from two distinct batches given that one product consisted of (±)-threo-4F-MPH isomers whereas the second sample consisted of a mixture of (±)-threo and (±)-erythro 4F-MPH. Monoamine transporter studies using rat brain synaptosomes revealed that the biological activity of the 4F-MPH mixture resided with the (±)-threo and not the (±)-erythro isomers based on higher potencies determined for blockage of dopamine uptake (IC50 4F-MPHmixture = 66 nM vs. IC50 (±)-threo = 61 nM vs. IC50 (±)-erythro = 8,528 nM) and norepinephrine uptake (IC50 4F-MPHmixture = 45 nM vs. (±)-threo = 31 nM vs. IC50 (±)-erythro = 3,779 nM). In comparison, MPH was three times less potent than (±)-threo-4F-MPH at the dopamine transporter (IC50 = 131 nM) and around 2.5 times less potent at the norepinephrine transporter (IC50 = 83 nM). Both substances were catecholamine selective with IC50 values of 8,805 nM and >10,000 nM for (±)-threo-4F-MPH and MPH at the serotonin transporter. These findings suggest that the psychostimulant properties of (±)-threo-4F-MPH might be more potent in humans than MPH. Copyright © 2017 John Wiley & Sons, Ltd.
Keywords: 4-fluoromethylphenidate; in vitro; monoamine transporters; new psychoactive substances; psychostimulants; methylphenidate.
Copyright © 2017 John Wiley & Sons, Ltd.
Figures


References
-
- Panizzon L. La preparazione di piridil- e piperidil-arilacetonitrili e di alcuni prodotti di trasformazione (Parte I) Helv Chim Acta. 1946;29:324.
-
- Meier R, Gross F, Tripod J. Ritalin, eine neuartige synthetische Verbindung mit spezifischer zentralerregender Wirkungskomponente. Klin Wochenschr. 1954;32:445. - PubMed
-
- Stahl M. Stahl's Essential Psychopharmacology: Neuroscientific Basis and Practical Applications, 4th edition. Cambridge University Press; Cambridge, UK: 2013.
-
- Deutsch HM, Shi Q, Gruszecka-Kowalik E, Schweri MM. Synthesis and pharmacology of potential cocaine antagonists. 2. Structure-activity relationship studies of aromatic ring-substituted methylphenidate analogs. J Med Chem. 1996;39:1201. - PubMed
-
- Malenka RC, Nestler EJ, Hyman SE. Chapter 6: Widely Projecting Systems: Monoamines, Acetylcholine, Orexin. In: Sydor A, Brown RY, editors. Molecular Neuropharmacology: A Foundation of Clinical Neuroscience. 2. McGraw-Hill Medical; New York: 2009.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

