ChMob2 binds to ChCbk1 and promotes virulence and conidiation of the fungal pathogen Colletotrichum higginsianum
- PMID: 28103800
- PMCID: PMC5248491
- DOI: 10.1186/s12866-017-0932-7
ChMob2 binds to ChCbk1 and promotes virulence and conidiation of the fungal pathogen Colletotrichum higginsianum
Abstract
Background: Mob family proteins are conserved between animals, plants and fungi and are essential for the activation of NDR kinases that control crucial cellular processes like cytokinesis, proliferation and morphology.
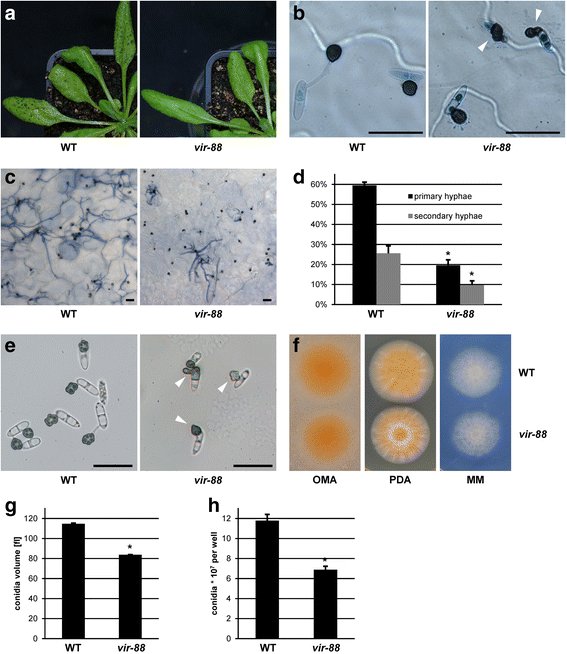
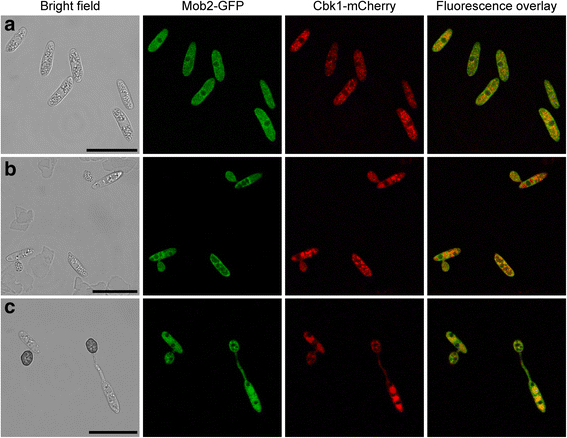
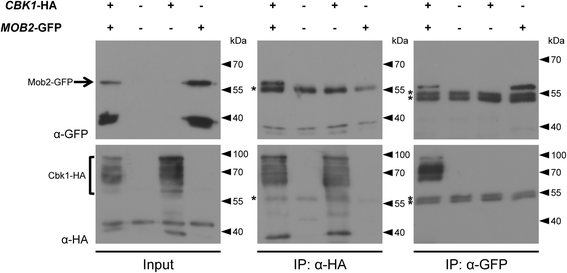
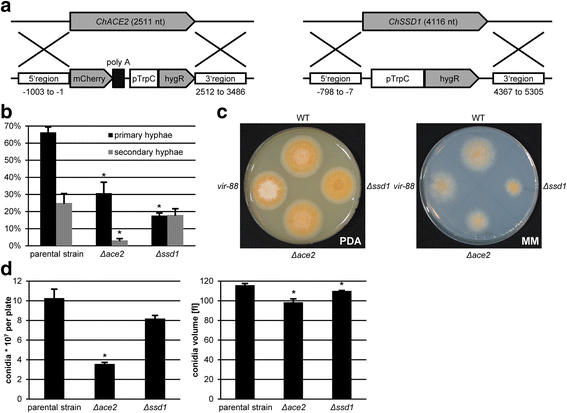
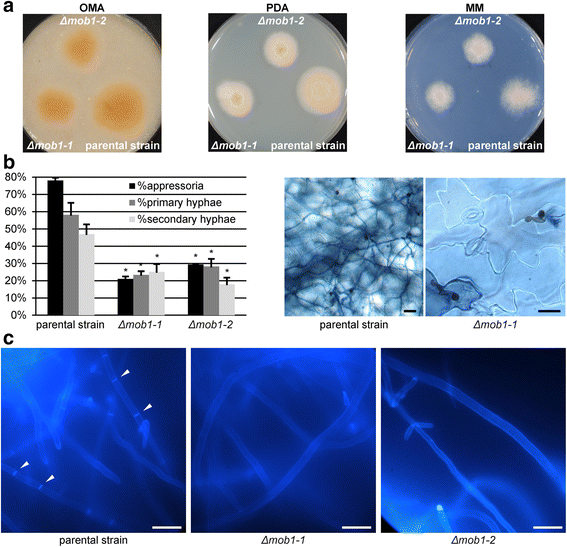
Results: We identified a hypomorphic allele of ChMOB2 in a random insertional mutant (vir-88) of the hemibiotrophic ascomycete fungus Colletotrichum higginsianum. The mutant is impaired in conidiation, host penetration and virulence on Arabidopsis thaliana. ChMob2 binds to and co-localizes with the NDR/LATS kinase homolog ChCbk1. Mutants in the two potential ChCbk1 downstream targets ChSSD1 and ChACE2 show defects in pathogenicity. The genome of C. higginsianum encodes two more Mob proteins. While we could not detect any effect on pathogenicity in ΔChmob3 mutants, ChMob1 is involved in conidiation, septae formation and virulence.
Conclusion: This study shows that ChMob2 binds to the conserved NDR/LATS Kinase ChCbk1 and plays an important role in pathogenicity of Colletotrichum higginsianum on Arabidopsis thaliana.
Keywords: ATMT; Ace2; Colletotrichum higginsianum; Conidiation; Cts1; Mob1; Mob2; Mob3; NDR Kinase Cbk1; Phytopathogenic ascomycete fungus; Ssd1.
Figures


Similar articles
-
Identification of virulence genes in the crucifer anthracnose fungus Colletotrichum higginsianum by insertional mutagenesis.Microb Pathog. 2013 Nov;64:6-17. doi: 10.1016/j.micpath.2013.06.001. Epub 2013 Jun 24. Microb Pathog. 2013. PMID: 23806215
-
Multifaceted Roles of the Ras Guanine-Nucleotide Exchange Factor ChRgf in Development, Pathogenesis, and Stress Responses of Colletotrichum higginsianum.Phytopathology. 2017 Apr;107(4):433-443. doi: 10.1094/PHYTO-03-16-0137-R. Epub 2017 Feb 15. Phytopathology. 2017. PMID: 28026997
-
A Genetic Screen for Pathogenicity Genes in the Hemibiotrophic Fungus Colletotrichum higginsianum Identifies the Plasma Membrane Proton Pump Pma2 Required for Host Penetration.PLoS One. 2015 May 19;10(5):e0125960. doi: 10.1371/journal.pone.0125960. eCollection 2015. PLoS One. 2015. PMID: 25992547 Free PMC article.
-
Colletotrichum higginsianum as a Model for Understanding Host⁻Pathogen Interactions: A Review.Int J Mol Sci. 2018 Jul 23;19(7):2142. doi: 10.3390/ijms19072142. Int J Mol Sci. 2018. PMID: 30041456 Free PMC article. Review.
-
The Role of Virulence Factors in the Pathogenicity of Colletotrichum sp.Curr Protein Pept Sci. 2017;18(10):1005-1018. doi: 10.2174/1389203717666160813160727. Curr Protein Pept Sci. 2017. PMID: 27526925 Review.
Cited by
-
When green and red mycology meet: Impressions from an interdisciplinary forum on virulence mechanisms of phyto- and human-pathogenic fungi.Virulence. 2017 Oct 3;8(7):1435-1444. doi: 10.1080/21505594.2017.1356502. Epub 2017 Jul 19. Virulence. 2017. PMID: 28723316 Free PMC article.
-
STRIPAK complex defects result in pseudosexual reproduction in Cryptococcus neoformans.bioRxiv [Preprint]. 2025 Apr 18:2025.04.08.647827. doi: 10.1101/2025.04.08.647827. bioRxiv. 2025. Update in: PLoS Genet. 2025 Jun 30;21(6):e1011774. doi: 10.1371/journal.pgen.1011774. PMID: 40297506 Free PMC article. Updated. Preprint.
-
STRIPAK complex defects result in pseudosexual reproduction in Cryptococcus neoformans.PLoS Genet. 2025 Jun 30;21(6):e1011774. doi: 10.1371/journal.pgen.1011774. eCollection 2025 Jun. PLoS Genet. 2025. PMID: 40587560 Free PMC article.
-
Hypothetical protein FoDbp40 influences the growth and virulence of Fusarium oxysporum by regulating the expression of isocitrate lyase.Front Microbiol. 2022 Nov 28;13:1050637. doi: 10.3389/fmicb.2022.1050637. eCollection 2022. Front Microbiol. 2022. PMID: 36519161 Free PMC article.
-
STRIPAK, a Key Regulator of Fungal Development, Operates as a Multifunctional Signaling Hub.J Fungi (Basel). 2021 Jun 1;7(6):443. doi: 10.3390/jof7060443. J Fungi (Basel). 2021. PMID: 34206073 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

