Sinularin induces DNA damage, G2/M phase arrest, and apoptosis in human hepatocellular carcinoma cells
- PMID: 28103869
- PMCID: PMC5248443
- DOI: 10.1186/s12906-017-1583-9
Sinularin induces DNA damage, G2/M phase arrest, and apoptosis in human hepatocellular carcinoma cells
Abstract
Background: Sinularin isolated from the cultured soft coral Sinularia flexibilis has been reported to exert potent cytotoxic effects against particular types of cancer. This study was carried out to investigate the cytotoxic effects in sinularin-treated human hepatocellular carcinoma cells, HepG2, and to subsequently explore the underlying molecular mechanisms.
Methods: TheMTT (3-[4,5-dimethylthiazol-2-yl]-2, 5-diphenyl- tetrazolium bromide) method was used to evaluate the cytotoxicity of sinularin on HepG2 and Hep3B cell lines. Furthermore, the cell cycle distribution assay, apoptosis assay, and western blot analysis in vitro were used to explore the possible mechanisms of action.
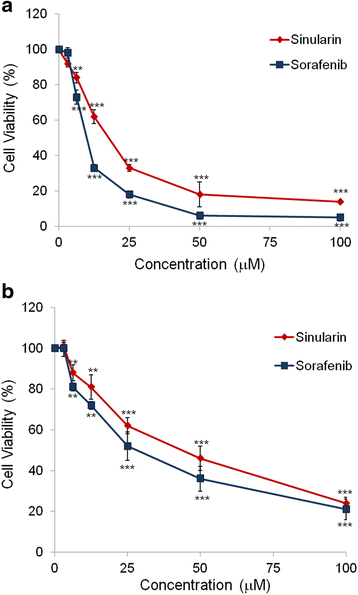
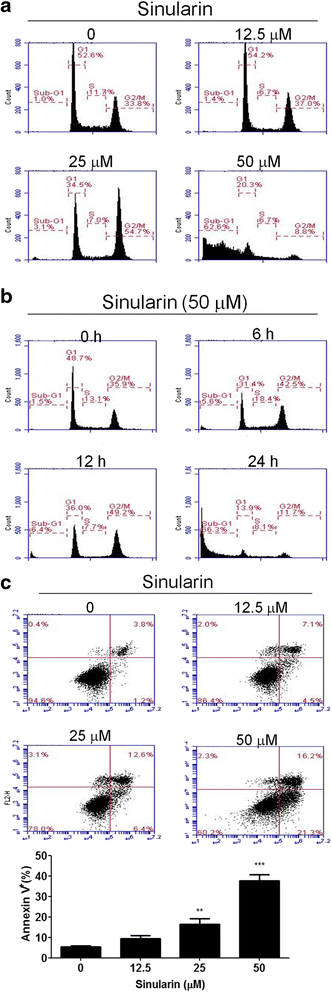
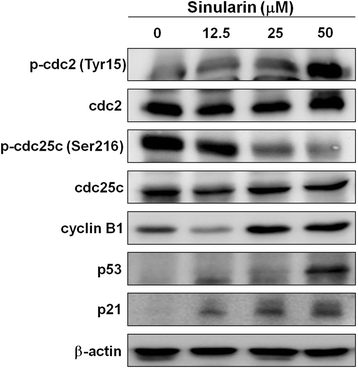
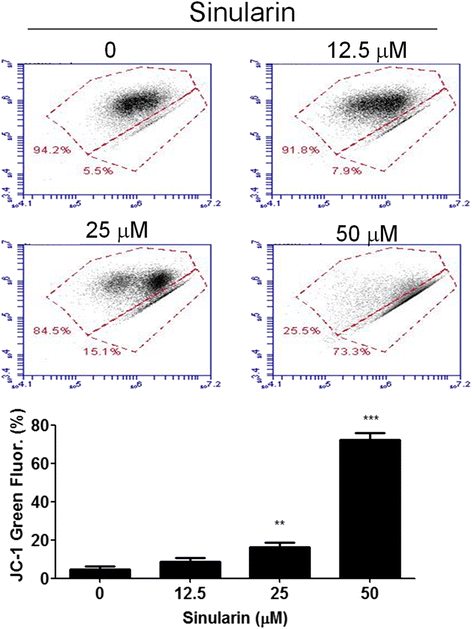
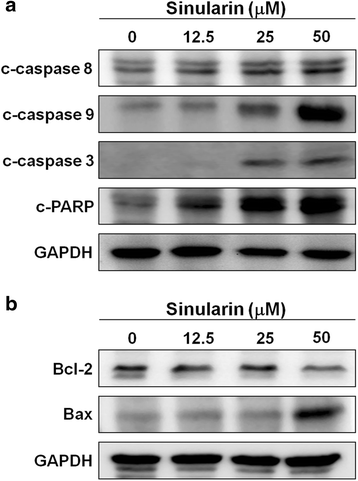
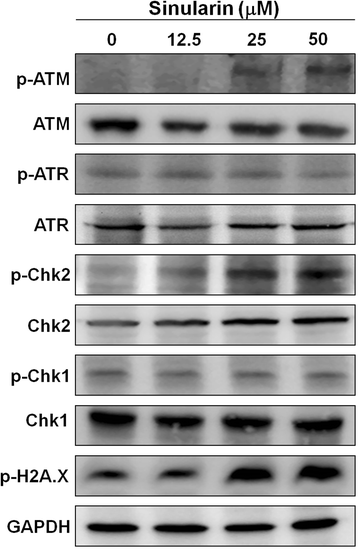
Results: From the results of our study, cell viability was obviously inhibited by sinularin in a dose-dependent manner. In addition, our results suggested that sinularin triggered DNA damage and subsequently induced cell cycle G2/M arrest associated with up-regulation of p-ATM (Ser(1981)), p-Chk2 (Tyr(68)), p-cdc2 (Tyr(15)), and p53 coupled with increased expression of downstream proteins p21 and down-regulation of p-cdc25 (Ser(216)). Moreover, the results of the apoptosis assay and western blot analysis indicated that the cytotoxic activity could be related to mitochondrial apoptosis, characterized by decrease of Bcl-2 expression, disruption of mitochondrial membrane potential, and sequential activation of caspases and Poly (ADP-ribose) polymerase (PARP).
Conclusions: This study reveals for the first time the anti-HCC activities of sinularin, the active compound isolated from the cultured soft coral Sinularia flexibilis. We believe that our results warrant further evaluation of sinularin as a new anti-HCC chemotherapeutic agent.
Keywords: Apoptosis; DNA damage; G2/M; Hepatocellular carcinoma; Sinularia flexibilis; Sinularin.
Figures
References
-
- Weinheimer AJ, Matson AJ, Hossain MB, van der Helm D. Marine anticancer agents: sinularin and dihydrosinularin, new cembranolides from the soft coral, sinularia flexibilis. Tetrahedron Lett. 1977;18:2923–6. doi: 10.1016/S0040-4039(01)83115-4. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

