Elevated collagen-I augments tumor progressive signals, intravasation and metastasis of prolactin-induced estrogen receptor alpha positive mammary tumor cells
- PMID: 28103936
- PMCID: PMC5244528
- DOI: 10.1186/s13058-017-0801-1
Elevated collagen-I augments tumor progressive signals, intravasation and metastasis of prolactin-induced estrogen receptor alpha positive mammary tumor cells
Abstract
Background: The development and progression of estrogen receptor alpha positive (ERα+) breast cancer has been linked epidemiologically to prolactin. However, activation of the canonical mediator of prolactin, STAT5, is associated with more differentiated cancers and better prognoses. We have reported that density/stiffness of the extracellular matrix potently modulates the repertoire of prolactin signals in human ERα + breast cancer cells in vitro: stiff matrices shift the balance from the Janus kinase (JAK)2/STAT5 cascade toward pro-tumor progressive extracellular regulated kinase (ERK)1/2 signals, driving invasion. However, the consequences for behavior of ERα + cancers in vivo are not known.
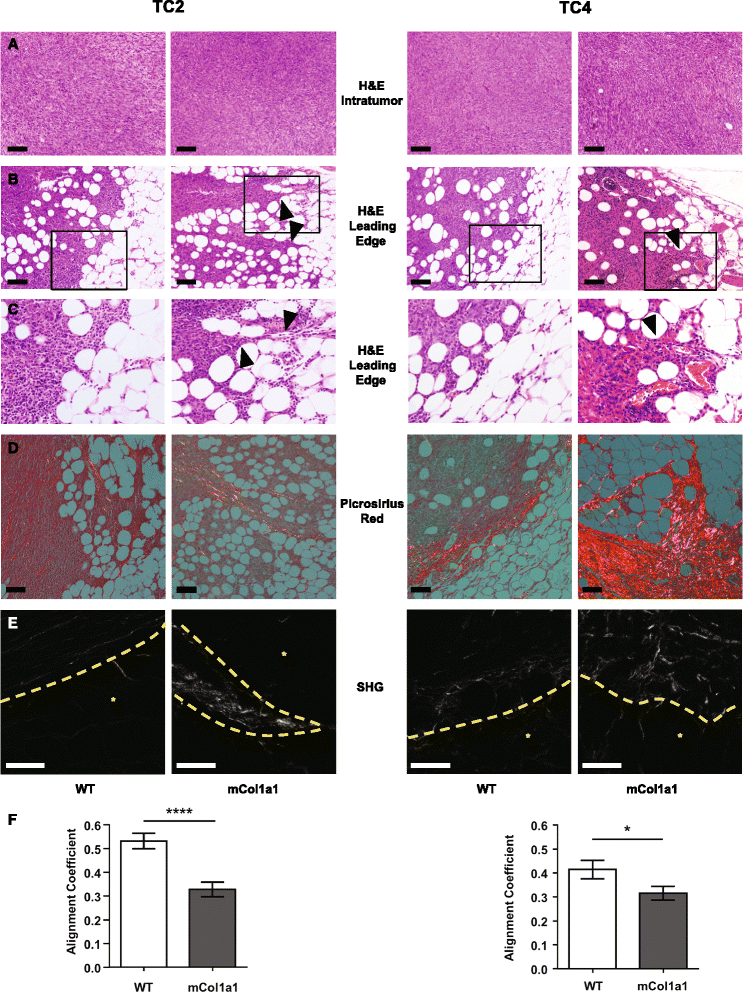
Methods: In order to investigate the importance of matrix density/stiffness in progression of ERα + cancers, we examined tumor development and progression following orthotopic transplantation of two clonal green fluorescent protein (GFP) + ERα + tumor cell lines derived from prolactin-induced tumors to 8-week-old wild-type FVB/N (WT) or collagen-dense (col1a1 tm1Jae/+ ) female mice. The latter express a mutant non-cleavable allele of collagen 1a1 "knocked-in" to the col1a1 gene locus, permitting COL1A1 accumulation. We evaluated the effect of the collagen environment on tumor progression by examining circulating tumor cells and lung metastases, activated signaling pathways by immunohistochemistry analysis and immunoblotting, and collagen structure by second harmonic generation microscopy.
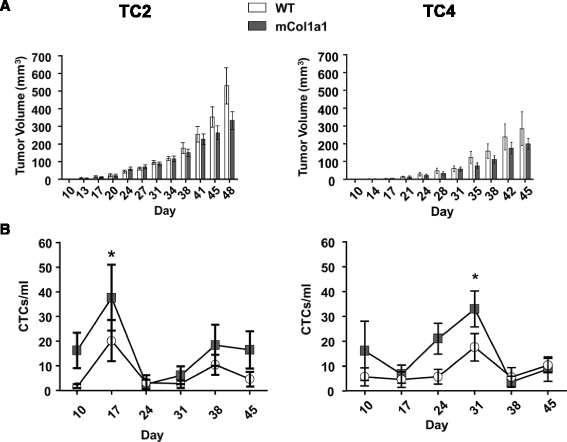
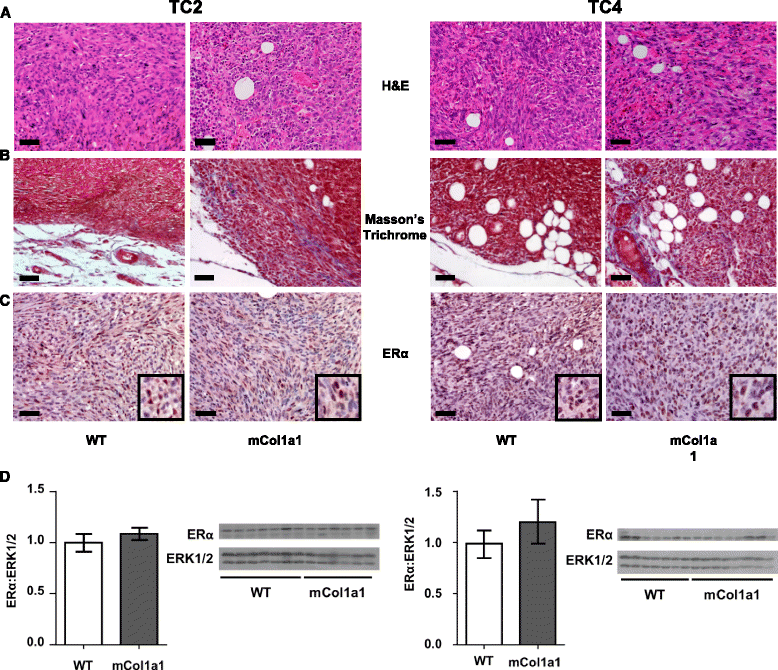
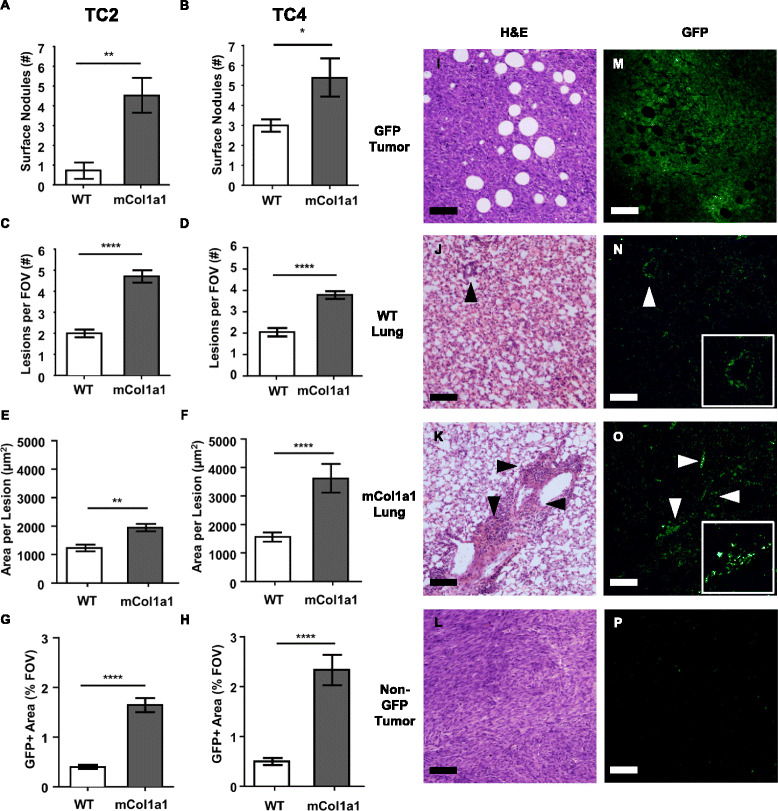
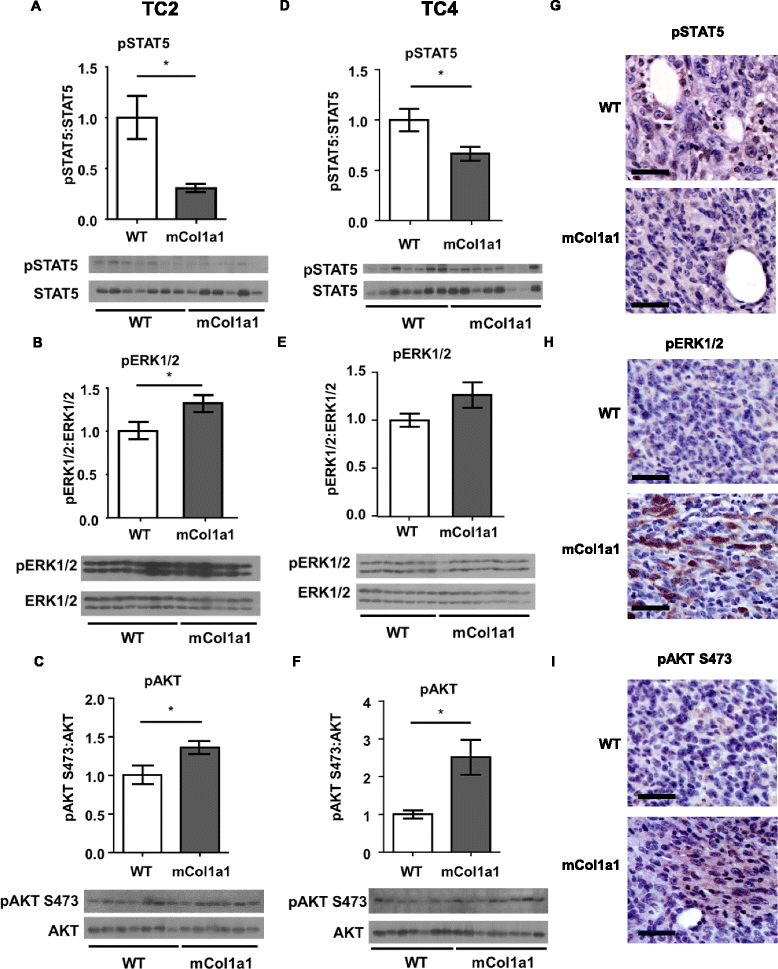
Results: ERα + primary tumors did not differ in growth rate, histologic type, ERα, or prolactin receptor (PRLR) expression between col1a1 tm1Jae/+ and WT recipients. However, the col1a1 tm1Jae/+ environment significantly increased circulating tumor cells and the number and size of lung metastases at end stage. Tumors in col1a1 tm1Jae/+ recipients displayed reduced STAT5 activation, and higher phosphorylation of ERK1/2 and AKT. Moreover, intratumoral collagen fibers in col1a1 tm1Jae/+ recipients were aligned with tumor projections into the adjacent fat pad, perpendicular to the bulk of the tumor, in contrast to the collagen fibers wrapped around the more uniformly expansive tumors in WT recipients.
Conclusions: A collagen-dense extracellular matrix can potently interact with hormonal signals to drive metastasis of ERα + breast cancers.
Keywords: Breast cancer; Collagen; Desmoplasia; Extracellular matrix; Prolactin; Tumor microenvironment; Tumor progression.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

