Allopregnanolone induces state-dependent fear via the bed nucleus of the stria terminalis
- PMID: 28104355
- PMCID: PMC5381271
- DOI: 10.1016/j.yhbeh.2017.01.002
Allopregnanolone induces state-dependent fear via the bed nucleus of the stria terminalis
Abstract
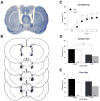
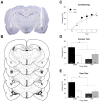
Gonadal steroids and their metabolites have been shown to be important modulators of emotional behavior. Allopregnanolone (ALLO), for example, is a metabolite of progesterone that has been linked to anxiety-related disorders such as posttraumatic stress disorder. In rodents, it has been shown to reduce anxiety in a number of behavioral paradigms including Pavlovian fear conditioning. We have recently found that expression of conditioned contextual (but not auditory) freezing in rats can be suppressed by infusion of ALLO into the bed nucleus of the stria terminalis (BNST). To further explore the nature of this effect, we infused ALLO into the BNST of male rats prior to both conditioning and testing. We found that suppression of contextual fear occurred when the hormone was present during either conditioning or testing but not during both procedures, suggesting that ALLO acts in a state-dependent manner within the BNST. A shift in interoceptive context during testing for animals conditioned under ALLO provided further support for this mechanism of hormonal action on contextual fear. Interestingly, infusions of ALLO into the basolateral amygdala produced a state-independent suppression of both conditioned contextual and auditory freezing. Altogether, these results suggest that ALLO can influence the acquisition and expression of fear memories by both state-dependent and state-independent mechanisms.
Keywords: Basolateral amygdala; Contextual fear; Fear conditioning; Neurosteroid.
Copyright © 2017 Elsevier Inc. All rights reserved.
Figures
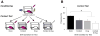
References
-
- Anagnostaras SG, Maren S, DeCola JP, Lane NI, Gale GD, Schlinger BA, Fanselow MS. Testicular hormones do not regulate sexually dimorphic Pavlovian fear conditioning or perforant-path long-term potentiation in adult male rats. Behav Brain Res. 1998;92:1–9. - PubMed
-
- Chang YJ, Yang CH, Liang YC, Yeh CM, Huang CC, Hsu KS. Estrogen modulates sexually dimorphic contextual fear extinction in rats through estrogen receptor β. Hippocampus. 2009;19:1142–1150. - PubMed
-
- Ebner DL, Richardson R, Riccio DC. Ovarian hormones and retention of learned fear in rats. Behav Neural Biol. 1981;33:45–58. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

