Correlation between Classic Driver Oncogene Mutations in EGFR, ALK, or ROS1 and 22C3-PD-L1 ≥50% Expression in Lung Adenocarcinoma
- PMID: 28104537
- PMCID: PMC5403565
- DOI: 10.1016/j.jtho.2016.12.026
Correlation between Classic Driver Oncogene Mutations in EGFR, ALK, or ROS1 and 22C3-PD-L1 ≥50% Expression in Lung Adenocarcinoma
Abstract
Introduction: Targeted somatic genomic analysis (EGFR, anaplastic lymphoma receptor tyrosine kinase gene [ALK], and ROS1) and programmed death ligand 1 (PD-L1) tumor proportion score (TPS) determined by immunohistochemistry (IHC) are used for selection of first-line therapies in advanced lung cancer; however, the frequency of overlap of these biomarkers in routine clinical practice is poorly reported.
Methods: We retrospectively probed the first 71 pairs of patients with lung adenocarcinoma from our institution. They were analyzed for PD-L1 by IHC using the clone 22C3 pharmDx kit (Agilent Technologies, Santa Clara, CA) and evaluated for co-occurrence of genomic aberrations and clinicopathologic characteristics.
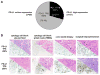
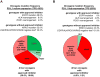
Results: Surgical resection specimens, small biopsy (transbronchial or core needle) samples, and cytologic cell blocks (needle aspirates or pleural fluid) were tested. A PD-L1 TPS of at least ≥50% was seen in 29.6% of tumors. Of 19 tumors with EGFR mutations, ALK fluorescence in situ hybridization positivity, or ROS1 fluorescence in situ hybridization positivity, 18 had a PD-L1 TPS less than 50% versus only one tumor with a PD-L1 TPS of at least 50% (p = 0.0073). Tumors with a PD-L1 TPS of at least 50% were significantly associated with smoking status compared with tumors with a PD-L1 TPS less than 50% but were not associated with patient sex, ethnicity, tumor stage, biopsy site, or biopsy type/preparation.
Conclusions: PD-L1 IHC can be performed on routine clinical lung cancer specimens. A TPS of at least 50% seldom overlaps with presence of driver oncogenes with approved targeted therapies. Three biomarker-specified groups of advanced lung adenocarcinomas can now be defined, each paired with a specific palliative first-line systemic therapy of proven clinical benefit: (1) EGFR/ALK/ROS1-affected adenocarcinoma paired with a matched tyrosine kinase inhibitor (∼20% of cases), (2) PD-L1-enriched adenocarcinoma (TPS ≥50%) paired with anti-PD-1 pembrolizumab (∼30% of cases), and (3) biomarker-negative (i.e., EGFR/ALK/ROS1/PD-L1-negative) adenocarcinoma paired with platinum doublet chemotherapy with or without bevacizumab (∼50% of cases).
Keywords: ALK; EGFR; Lung cancer; PD-1; PD-L1; Pembrolizumab.
Copyright © 2017 International Association for the Study of Lung Cancer. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures
Comment in
-
Correlation between 22C3-PD-L1 Expression and EGFR Mutations in Japanese Patients with Advanced Lung Adenocarcinoma.J Thorac Oncol. 2018 May;13(5):e79-e81. doi: 10.1016/j.jtho.2018.01.011. J Thorac Oncol. 2018. PMID: 29703543 No abstract available.
-
Updated Correlation of 22C3-PD-L1 ≥50% Expression with Driver Oncogene Mutations and Response to Pembrolizumab in the Kinase Inhibitor-Resistant Setting.J Thorac Oncol. 2018 May;13(5):e81-e83. doi: 10.1016/j.jtho.2018.01.023. J Thorac Oncol. 2018. PMID: 29703544 No abstract available.
References
-
- Reck M, Rodriguez-Abreu D, Robinson AG, et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N Engl J Med. 2016 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

