Mice with a deficiency in CLEC-2 are protected against deep vein thrombosis
- PMID: 28104688
- PMCID: PMC5408561
- DOI: 10.1182/blood-2016-09-742999
Mice with a deficiency in CLEC-2 are protected against deep vein thrombosis
Abstract
Deep vein thrombosis (DVT) with its major complication, pulmonary embolism, is a global health problem. Mechanisms of DVT remain incompletely understood. Platelets play a role in DVT, but the impact of specific platelet receptors remains unclear. Platelet C-type lectin-like receptor 2 (CLEC-2) is known to maintain the physiological state of blood vasculature under inflammatory conditions. DVT is a thromboinflammatory disorder developing largely as sterile inflammation in the vessel wall. We hypothesized therefore that CLEC-2 might play a role in DVT. Here, using a murine DVT model of inferior vena cava (IVC) stenosis, we demonstrate that mice with general inducible deletion of CLEC-2 or platelet-specific deficiency in CLEC-2 are protected against DVT. No phenotype in the complete stasis model was observed. Transfusion of wild-type platelets into platelet-specific CLEC-2 knockout mice restored thrombosis. Deficiency in CLEC-2 as well as inhibition of podoplanin, a ligand of CLEC-2, was associated with reduced platelet accumulation at the IVC wall after 6 hours of stenosis. Podoplanin was expressed in the IVC wall, where it was localized in the vicinity of the abluminal side of the endothelium. The level of podoplanin in the IVC increased after 48 hours of stenosis to a substantially higher extent in mice with a thrombus vs those without a thrombus. Treatment of animals with an anti-podoplanin neutralizing antibody resulted in development of smaller thrombi. Thus, we propose a novel mechanism of DVT, whereby CLEC-2 and upregulation of podoplanin expression in the venous wall trigger thrombus formation.
© 2017 by The American Society of Hematology.
Figures
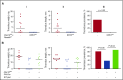
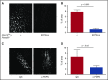
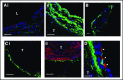


Comment in
-
CLEC-2/podoplanin and thromboinflammation.Blood. 2017 Apr 6;129(14):1896-1898. doi: 10.1182/blood-2017-02-764670. Blood. 2017. PMID: 28385772 No abstract available.
References
-
- May F, Hagedorn I, Pleines I, et al. . CLEC-2 is an essential platelet-activating receptor in hemostasis and thrombosis. Blood. 2009;114(16):3464-3472. - PubMed
-
- Suzuki-Inoue K, Inoue O, Ding G, et al. . Essential in vivo roles of the C-type lectin receptor CLEC-2: embryonic/neonatal lethality of CLEC-2-deficient mice by blood/lymphatic misconnections and impaired thrombus formation of CLEC-2-deficient platelets. J Biol Chem. 2010;285(32):24494-24507. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

