Clinical targeted exome-based sequencing in combination with genome-wide copy number profiling: precision medicine analysis of 203 pediatric brain tumors
- PMID: 28104717
- PMCID: PMC5570190
- DOI: 10.1093/neuonc/now294
Clinical targeted exome-based sequencing in combination with genome-wide copy number profiling: precision medicine analysis of 203 pediatric brain tumors
Abstract
Background: Clinical genomics platforms are needed to identify targetable alterations, but implementation of these technologies and best practices in routine clinical pediatric oncology practice are not yet well established.
Methods: Profile is an institution-wide prospective clinical research initiative that uses targeted sequencing to identify targetable alterations in tumors. OncoPanel, a multiplexed targeted exome-sequencing platform that includes 300 cancer-causing genes, was used to assess single nucleotide variants and rearrangements/indels. Alterations were annotated (Tiers 1-4) based on clinical significance, with Tier 1 alterations having well-established clinical utility. OncoCopy, a clinical genome-wide array comparative genomic hybridization (aCGH) assay, was also performed to evaluate copy number alterations and better define rearrangement breakpoints.
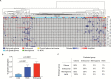
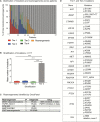
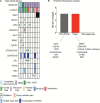
Results: Cancer genomes of 203 pediatric brain tumors were profiled across histological subtypes, including 117 samples analyzed by OncoPanel, 146 by OncoCopy, and 60 tumors subjected to both methodologies. OncoPanel revealed clinically relevant alterations in 56% of patients (44 cancer mutations and 20 rearrangements), including BRAF alterations that directed the use of targeted inhibitors. Rearrangements in MYB-QKI, MYBL1, BRAF, and FGFR1 were also detected. Furthermore, while copy number profiles differed across histologies, the combined use of OncoPanel and OncoCopy identified subgroup-specific alterations in 89% (17/19) of medulloblastomas.
Conclusion: The combination of OncoPanel and OncoCopy multiplex genomic assays can identify critical diagnostic, prognostic, and treatment-relevant alterations and represents an effective precision medicine approach for clinical evaluation of pediatric brain tumors.
Keywords: array CGH; brain tumor; clinical sequencing; pediatric neuro-oncology; precision medicine.
© The Author(s) 2017. Published by Oxford University Press on behalf of the Society for Neuro-Oncology. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com
Figures


References
-
- Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2012. National Cancer Institute.
-
- Oeffinger KC, Mertens AC, Sklar CA, et al. Chronic health conditions in adult survivors of childhood cancer. N Engl J Med. 2006;355(15):1572–1582. doi:10.1056/NEJMsa060185. - PubMed
-
- Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417(6892):949–954. doi:10.1038/nature00766. - PubMed
-
- Takeuchi K, Soda M, Togashi Y, et al. RET, ROS1 and ALK fusions in lung cancer. Nat Med. 2012;18(3):378–381. doi:10.1038/nm.2658. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

