Exosomes Generated From iPSC-Derivatives: New Direction for Stem Cell Therapy in Human Heart Diseases
- PMID: 28104773
- PMCID: PMC5260934
- DOI: 10.1161/CIRCRESAHA.116.309307
Exosomes Generated From iPSC-Derivatives: New Direction for Stem Cell Therapy in Human Heart Diseases
Abstract
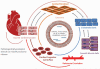
Cardiovascular disease (CVD) is the leading cause of death in modern society. The adult heart innately lacks the capacity to repair and regenerate the damaged myocardium from ischemic injury. Limited understanding of cardiac tissue repair process hampers the development of effective therapeutic solutions to treat CVD such as ischemic cardiomyopathy. In recent years, rapid emergence of induced pluripotent stem cells (iPSC) and iPSC-derived cardiomyocytes presents a valuable opportunity to replenish the functional cells to the heart. The therapeutic effects of iPSC-derived cells have been investigated in many preclinical studies. However, the underlying mechanisms of iPSC-derived cell therapy are still unclear, and limited engraftment of iPSC-derived cardiomyocytes is well known. One facet of their mechanism is the paracrine effect of the transplanted cells. Microvesicles such as exosomes secreted from the iPSC-derived cardiomyocytes exert protective effects by transferring the endogenous molecules to salvage the injured neighboring cells by regulating apoptosis, inflammation, fibrosis, and angiogenesis. In this review, we will focus on the current advances in the exosomes from iPSC derivatives and discuss their therapeutic potential in the treatment of CVD.
Keywords: cardiovascular disease; cell-free personalized medicine; exosomes; induced pluripotent stem cells; intercellular communication.
© 2017 American Heart Association, Inc.
Figures


References
-
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. - PubMed
-
- Okano H, Nakamura M, Yoshida K, Okada Y, Tsuji O, Nori S, Ikeda E, Yamanaka S, Miura K. Steps toward safe cell therapy using induced pluripotent stem cells. Circulation Research. 2013;112:523–533. - PubMed
-
- Shiba Y, Gomibuchi T, Seto T, Wada Y, Ichimura H, Tanaka Y, Ogasawara T, Okada K, Shiba N, Sakamoto K, Ido D, Shiina T, Ohkura M, Nakai J, Uno N, et al. Allogeneic transplantation of iPS cell-derived cardiomyocytes regenerates primate hearts. Nature. 2016;538:388–391. - PubMed
-
- Beltrami AP, Barlucchi L, Torella D, Baker M, Limana F, Chimenti S, Kasahara H, Rota M, Musso E, Urbanek K, Leri A, Kajstura J, Nadal-Ginard B, Anversa P. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114:763–776. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

