Antibody-secreting plasma cells persist for decades in human intestine
- PMID: 28104812
- PMCID: PMC5294861
- DOI: 10.1084/jem.20161590
Antibody-secreting plasma cells persist for decades in human intestine
Abstract
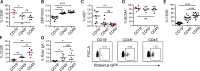
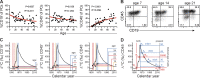
Plasma cells (PCs) produce antibodies that mediate immunity after infection or vaccination. In contrast to PCs in the bone marrow, PCs in the gut have been considered short lived. In this study, we studied PC dynamics in the human small intestine by cell-turnover analysis in organ transplants and by retrospective cell birth dating measuring carbon-14 in genomic DNA. We identified three distinct PC subsets: a CD19+ PC subset was dynamically exchanged, whereas of two CD19- PC subsets, CD45+ PCs exhibited little and CD45- PCs no replacement and had a median age of 11 and 22 yr, respectively. Accumulation of CD45- PCs during ageing and the presence of rotavirus-specific clones entirely within the CD19- PC subsets support selection and maintenance of protective PCs for life in human intestine.
© 2017 Landsverk et al.
Figures



References
-
- Bhoj, V.G., Arhontoulis D., Wertheim G., Capobianchi J., Callahan C.A., Ellebrecht C.T., Obstfeld A.E., Lacey S.F., Melenhorst J.J., Nazimuddin F., et al. . 2016. Persistence of long-lived plasma cells and humoral immunity in individuals responding to CD19-directed CAR T-cell therapy. Blood. 128:360–370. 10.1182/blood-2016-01-694356 - DOI - PMC - PubMed
-
- Di Niro, R., Mesin L., Raki M., Zheng N.Y., Lund-Johansen F., Lundin K.E., Charpilienne A., Poncet D., Wilson P.C., and Sollid L.M.. 2010. Rapid generation of rotavirus-specific human monoclonal antibodies from small-intestinal mucosa. J. Immunol. 185:5377–5383. 10.4049/jimmunol.1001587 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

