Selective neuronal vulnerability in Parkinson disease
- PMID: 28104909
- PMCID: PMC5564322
- DOI: 10.1038/nrn.2016.178
Selective neuronal vulnerability in Parkinson disease
Abstract
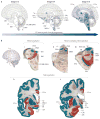
Intracellular α-synuclein (α-syn)-rich protein aggregates called Lewy pathology (LP) and neuronal death are commonly found in the brains of patients with clinical Parkinson disease (cPD). It is widely believed that LP appears early in the disease and spreads in synaptically coupled brain networks, driving neuronal dysfunction and death. However, post-mortem analysis of human brains and connectome-mapping studies show that the pattern of LP in cPD is not consistent with this simple model, arguing that, if LP propagates in cPD, it must be gated by cell- or region-autonomous mechanisms. Moreover, the correlation between LP and neuronal death is weak. In this Review, we briefly discuss the evidence for and against the spreading LP model, as well as evidence that cell-autonomous factors govern both α-syn pathology and neuronal death.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Berg D, et al. Time to redefine PD? Introductory statement of the MDS task force on the definition of Parkinson’s disease. Mov Disord. 2014;29:454–462. This article reviews clinical and biological knowledge on PD showing that LP is not essential for cPD and occurs in a large proportion of patients who may not have cPD. - PMC - PubMed
-
- Hornykiewicz O. Dopamine miracle: from brain homogenate to dopamine replacement. Mov Disord. 2002;17:501–508. - PubMed
-
- Goedert M, Spillantini MG, Del Tredici K, Braak H. 100 years of Lewy pathology. Nat Rev Neurol. 2012;9:13–24. - PubMed
-
- Dijkstra AA, et al. Stage-dependent nigral neuronal loss in incidental Lewy body and Parkinson’s disease. Mov Disord. 2014;29:1244–1251. This paper describes a negative correlation between neuronal density and local α-syn burden in the SN of patients with PD and shows that the severity of neurodegeneration and local burden of α-syn pathological conditions are closely coupled during disease progression. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

