Neuronal Cell Bodies Remotely Regulate Axonal Growth Response to Localized Netrin-1 Treatment via Second Messenger and DCC Dynamics
- PMID: 28105005
- PMCID: PMC5214882
- DOI: 10.3389/fncel.2016.00298
Neuronal Cell Bodies Remotely Regulate Axonal Growth Response to Localized Netrin-1 Treatment via Second Messenger and DCC Dynamics
Abstract
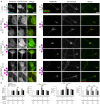
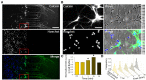
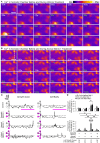
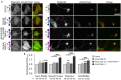
Netrin-1 modulates axonal growth direction and speed. Its best characterized receptor, Deleted in Colorectal Cancer (DCC), is localized to growth cones, but also observed in the cell bodies. We hypothesized that cell bodies sense Netrin-1 and contribute to axon growth rate modulation, mediated by the second messenger system. We cultured mouse cortical neurons in microfluidic devices to isolate distal axon and cell body microenvironments. Compared to isolated axonal treatment, global Netrin-1 treatment decreased the axon elongation rate and affected the dynamics of total and membranous DCC, calcium, and cyclic nucleotides. Signals induced by locally applied Netrin-1 propagated in both anterograde and retrograde directions, demonstrated by the long-range increase in DCC and by the increased frequency of calcium transients in cell bodies, evoked by axonal Netrin-1. Blocking the calcium efflux from endoplasmic reticulum suppressed the membranous DCC response. Our findings support the notion that neurons sense Netrin-1 along their entire lengths in making axonal growth decisions.
Keywords: calcium signaling; compartmentalization; guidance cues; microfluidics; pathfinding.
Figures



Similar articles
-
Neuron Subpopulations with Different Elongation Rates and DCC Dynamics Exhibit Distinct Responses to Isolated Netrin-1 Treatment.ACS Chem Neurosci. 2015 Sep 16;6(9):1578-90. doi: 10.1021/acschemneuro.5b00142. Epub 2015 Jul 31. ACS Chem Neurosci. 2015. PMID: 26190161
-
Coordinated interaction of Down syndrome cell adhesion molecule and deleted in colorectal cancer with dynamic TUBB3 mediates Netrin-1-induced axon branching.Neuroscience. 2015 May 7;293:109-22. doi: 10.1016/j.neuroscience.2015.02.042. Epub 2015 Mar 6. Neuroscience. 2015. PMID: 25754961 Free PMC article.
-
The calcium-activated protease calpain regulates netrin-1 receptor deleted in colorectal cancer-induced axon outgrowth in cortical neurons.J Neurochem. 2020 Feb;152(3):315-332. doi: 10.1111/jnc.14837. Epub 2019 Aug 22. J Neurochem. 2020. PMID: 31344270
-
The Deleted in Colorectal Cancer netrin guidance system: a molecular strategy for neuronal navigation.Clin Exp Pharmacol Physiol. 1999 Sep;26(9):749-51. doi: 10.1046/j.1440-1681.1999.03106.x. Clin Exp Pharmacol Physiol. 1999. PMID: 10499167 Review.
-
The dependence receptors DCC and UNC5H as a link between neuronal guidance and survival.Biol Cell. 2003 Oct;95(7):425-36. doi: 10.1016/s0248-4900(03)00072-8. Biol Cell. 2003. PMID: 14597260 Review.
Cited by
-
The Emerging Role of Mechanics in Synapse Formation and Plasticity.Front Cell Neurosci. 2018 Dec 6;12:483. doi: 10.3389/fncel.2018.00483. eCollection 2018. Front Cell Neurosci. 2018. PMID: 30574071 Free PMC article. Review.
-
DCC mutation update: Congenital mirror movements, isolated agenesis of the corpus callosum, and developmental split brain syndrome.Hum Mutat. 2018 Jan;39(1):23-39. doi: 10.1002/humu.23361. Epub 2017 Nov 11. Hum Mutat. 2018. PMID: 29068161 Free PMC article. Review.
-
Subcellular electrical stimulation of neurons enhances the myelination of axons by oligodendrocytes.PLoS One. 2017 Jul 3;12(7):e0179642. doi: 10.1371/journal.pone.0179642. eCollection 2017. PLoS One. 2017. PMID: 28671962 Free PMC article.
-
Microfluidic Multielectrode Arrays for Spatially Localized Drug Delivery and Electrical Recordings of Primary Neuronal Cultures.Front Bioeng Biotechnol. 2020 Jun 16;8:626. doi: 10.3389/fbioe.2020.00626. eCollection 2020. Front Bioeng Biotechnol. 2020. PMID: 32656200 Free PMC article.
-
Houshiheisan and its components promote axon regeneration after ischemic brain injury.Neural Regen Res. 2018 Jul;13(7):1195-1203. doi: 10.4103/1673-5374.235031. Neural Regen Res. 2018. PMID: 30028327 Free PMC article.
References
-
- Bartoe J. L., McKenna W. L., Quan T. K., Stafford B. K., Moore J. A., Xia J., et al. . (2006). Protein interacting with C-kinase 1/protein kinase Cα-mediated endocytosis converts netrin-1-mediated repulsion to attraction. J. Neurosci. 26, 3192–3205. 10.1523/JNEUROSCI.3469-05.2006 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

