The Inhibitory Effects of Nesfatin-1 in Ventromedial Hypothalamus on Gastric Function and Its Regulation by Nucleus Accumbens
- PMID: 28105016
- PMCID: PMC5213809
- DOI: 10.3389/fphys.2016.00634
The Inhibitory Effects of Nesfatin-1 in Ventromedial Hypothalamus on Gastric Function and Its Regulation by Nucleus Accumbens
Abstract
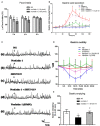
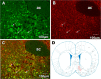
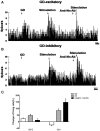
Aim: The aim of this study was to investigate the effect of nesfatin-1 signaling in the ventromedial hypothalamus (VMH) on gastric functions, as well as the regulation of these effects by nucleus accumbens (NAc) projections to VMH. Methods: The expression of c-fos in nesfatinergic VMH neurons induced by gastric distension (GD) was measured using the double fluoro-immunohistochemical staining. The firing rates of neurons were monitored with single-unit extracellular electric discharge recording. The projection of nesfatinergic neurons from NAc to VMH was observed by fluorogold retrograde tracer combined with fluoro-immunohistochemical staining. The effect of nesfatin-1 in VMH or electric stimulation in NAc on gastric function was studied by measuring food intake, gastric acid output, gastric motility, and gastric emptying, and the ability of the melanocortin-3/4 receptor antagonist SHU9119 or the anti-nesfatin-1 antibody to block nesfatin-1 in the VMH was assessed. Results: Expression of c-fos was observed in VMH nesfatinergic neurons following GD in rats. Further, nesfatin-1 delivery to single GD-responsive neurons changed the firing rates of these neurons in the VMH. In awake, behaving rats, intra-VMH administration of nesfatin-1 inhibited food intake, gastric acid output, gastric motility, and gastric emptying. These effects were abolished by SHU9119. Fluorogold retrograde tracing showed nesfatinergic neural projection from the NAc to the VMH. Electrical stimulation of NAc modified the firing rates of the VMH neurons and inhibited food intake and gastric functions. The pretreatment with an anti-nesfatin-1 antibody in the VMH reversed the effects of NAc electrical stimulation on the VMH neuronal firing rates and gastric function. Conclusions: Nesfatin-1 in the VMH inhibited food intake, gastric acid output, gastric motility, and gastric emptying. A nesfatinergic pathway between NAc and VMH transmitted metabolism-regulating signals.
Keywords: food intake; gastric function; nesfatin-1; nucleus accumbens; ventromedial hypothalamus.
Figures




References
-
- Duva M. A., Tomkins E. M., Moranda L. M., Kaplan R., Sukhaseum A., Stanley B. G. (2005). Origins of lateral hypothalamic afferents associated with N-methyl-d-aspartic acid-elicited eating studied using reverse microdialysis of NMDA and Fluorogold. Neurosci. Res. 52, 95–106. 10.1016/j.neures.2005.02.001 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

