Silencing of hypoxia-inducible factor-1α promotes thyroid cancer cell apoptosis and inhibits invasion by downregulating WWP2, WWP9, VEGF and VEGFR2
- PMID: 28105105
- PMCID: PMC5228437
- DOI: 10.3892/etm.2016.3826
Silencing of hypoxia-inducible factor-1α promotes thyroid cancer cell apoptosis and inhibits invasion by downregulating WWP2, WWP9, VEGF and VEGFR2
Abstract
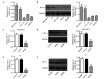
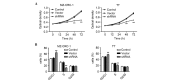
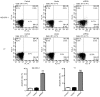
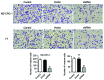
Adaptation to hypoxia is an important process physiologically and pathologically. Hypoxia-inducible factor-1α (HIF-1α) participates in the cancer biology of numerous endocrine tumors, including their proliferation and differentiation. In the present study, the hypothesis that HIF-1α promotes tumorigenesis in thyroid cancer via upregulating angiogenesis-associated markers is investigated. Reverse transcription-quantitative polymerase chain reaction (RT-qPCR) and western blot analysis were used to examine the expression of HIF-1α in thyroid cancer cell lines, and to detect the expression of WW domain containing E3 ubiquitin protein ligase (WWP)2, WWP9, vascular endothelial growth factor (VEGF) and VEGF receptor 2 (VEGFR2) in MZ-CRC-1 and TT thyroid cancer cells. Cell proliferation was measured using a Cell Count Kit-8. Cell apoptosis and cell cycle was assessed by flow cytometry. Cell invasive ability was examined by Matrigel transwell analysis. RT-qPCR and western blot analyses demonstrated that the mRNA and protein expression levels of HIF-1α were significant higher in MZ-CRC-1 and TT thyroid cancer cells than in another three thyroid cancer cells (P<0.01). HIF-1α knockdown cells demonstrated inhibition of cell proliferation and invasion, arrested cell cycle at the G1 phase, and induction of cell apoptosis. The protein expression levels of WWP2, WWP9, VEGF and VEGFR2 were decreased in HIF-1α knockdown MZ-CRC-1 and TT cells. In conclusion, HIF-1α may be important in cell apoptosis and invasion of thyroid cancer cells, likely through regulating WWP2, WWP9, VEGF and VEGFR2 expression.
Keywords: angiogenesis; hypoxia; hypoxia-inducible factor-1α; invasion; thyroid cancer.
Figures

Similar articles
-
Centchroman regulates breast cancer angiogenesis via inhibition of HIF-1α/VEGFR2 signalling axis.Life Sci. 2018 Jan 15;193:9-19. doi: 10.1016/j.lfs.2017.11.045. Epub 2017 Nov 28. Life Sci. 2018. PMID: 29196053
-
Knockdown of hypoxia-inducible factor-1 alpha reduces proliferation, induces apoptosis and attenuates the aggressive phenotype of retinoblastoma WERI-Rb-1 cells under hypoxic conditions.Ann Clin Lab Sci. 2014 Spring;44(2):134-44. Ann Clin Lab Sci. 2014. PMID: 24795051
-
Magnolol suppresses hypoxia-induced angiogenesis via inhibition of HIF-1α/VEGF signaling pathway in human bladder cancer cells.Biochem Pharmacol. 2013 May 1;85(9):1278-87. doi: 10.1016/j.bcp.2013.02.009. Epub 2013 Feb 14. Biochem Pharmacol. 2013. PMID: 23416116
-
Chlorogenic acid inhibits hypoxia-induced angiogenesis via down-regulation of the HIF-1α/AKT pathway.Cell Oncol (Dordr). 2015 Apr;38(2):111-8. doi: 10.1007/s13402-014-0216-2. Epub 2015 Jan 6. Cell Oncol (Dordr). 2015. PMID: 25561311
-
Progress on the HIF-1α/VEGF/VEGFR2 signal pathway in hepatic alveolar echinococcosis.Front Oncol. 2025 Apr 8;15:1553125. doi: 10.3389/fonc.2025.1553125. eCollection 2025. Front Oncol. 2025. PMID: 40265025 Free PMC article. Review.
Cited by
-
Mulberry anthocyanins improves thyroid cancer progression mainly by inducing apoptosis and autophagy cell death.Kaohsiung J Med Sci. 2018 May;34(5):255-262. doi: 10.1016/j.kjms.2017.11.004. Epub 2017 Dec 6. Kaohsiung J Med Sci. 2018. PMID: 29699632 Free PMC article.
-
Etomidate inhibits cell proliferation and induces apoptosis in A549 non-small cell lung cancer cells via downregulating WWP2.Exp Ther Med. 2021 Nov;22(5):1254. doi: 10.3892/etm.2021.10689. Epub 2021 Sep 3. Exp Ther Med. 2021. PMID: 34603522 Free PMC article.
-
Hypoxia signaling pathway: A central mediator in endocrine tumors.Front Endocrinol (Lausanne). 2023 Jan 9;13:1103075. doi: 10.3389/fendo.2022.1103075. eCollection 2022. Front Endocrinol (Lausanne). 2023. PMID: 36699028 Free PMC article. Review.
-
GPCRs in Cancer: Protease-Activated Receptors, Endocytic Adaptors and Signaling.Int J Mol Sci. 2018 Jun 27;19(7):1886. doi: 10.3390/ijms19071886. Int J Mol Sci. 2018. PMID: 29954076 Free PMC article. Review.
-
Monocarboxylate transporter 1 (MCT1), a tool to stratify acute myeloid leukemia (AML) patients and a vehicle to kill cancer cells.Oncotarget. 2017 Aug 16;8(47):82803-82823. doi: 10.18632/oncotarget.20294. eCollection 2017 Oct 10. Oncotarget. 2017. PMID: 29137304 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
