Upregulation of microRNA-181b inhibits CCL18-induced breast cancer cell metastasis and invasion via the NF-κB signaling pathway
- PMID: 28105154
- PMCID: PMC5228575
- DOI: 10.3892/ol.2016.5230
Upregulation of microRNA-181b inhibits CCL18-induced breast cancer cell metastasis and invasion via the NF-κB signaling pathway
Retraction in
-
[Retracted] Upregulation of microRNA‑181b inhibits CCL18‑induced breast cancer cell metastasis and invasion via the NF‑κB signaling pathway.Oncol Lett. 2024 Apr 15;27(6):268. doi: 10.3892/ol.2024.14401. eCollection 2024 Jun. Oncol Lett. 2024. PMID: 38659419 Free PMC article.
Abstract
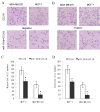
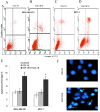
The purpose of the present study was to investigate the effects of upregulating microRNA (miR)-181b expression in tumor-associated macrophages regarding breast cancer cell metastasis and to identify the target gene. Ectopic miR-181b was transfected into MDA-MB-231 and MCF-7 breast cancer cell lines with or without chemokine ligand 18 (CCL18) stimulation. Cell proliferation, migration/invasion and apoptosis rate were investigated. The binding effects of miR-181b to the 3'-untranslated region (UTR) of the nuclear factor (NF)-κB gene were detected with the dual luciferase reporter system. Immunofluorescent staining of the NF-κB key component P65 was performed. The messenger (m) RNA and protein expression of NF-κB induced by CCL18 with or without miR-181b stimulation was evaluated with reverse transcription-quantitative polymerase chain reaction and western blot analysis. When compared with the CCL18-stimulated group, miR-181b mimic-transfected cells exhibited significantly inhibited proliferation and migration, with an increased cell apoptosis percentage in a dose-dependent manner. Furthermore, the luciferase activity was reduced for cells with NF-κB 3'-UTR wild-type that were co-transfected with miR-181b mimics. Immunofluorescent staining of NF-κB demonstrably weakened the P65 signal in stimulated miR-181b mimic cells when compared with parental and CCL18-treated cells. The increased expression level of NF-κB induced by CCL18 in MDA-MB-231 and MCF-7 cells was suppressed by miR-181b mimics. Overexpression of miR-181b suppressed cell survival rate and migration. This overexpression may achieve this goal by regulating the NF-κB pathway in breast cancer cells. Our study demonstrated a potential therapeutic application of miR-181b in the treatment of breast cancer.
Keywords: apoptosis; breast cancer cell; chemokine ligand 18; microRNAs; migration.
Figures


References
-
- Chang CY, Lee YH, Leu SJ, Wang CY, Wei CP, Hung KS, Pai MH, Tsai MD, Wu CH. CC-chemokine ligand 18/pulmonary activation-regulated chemokine expression in the CNS with special reference to traumatic brain injuries and neoplastic disorders. Neuroscience. 2010;165:1233–1243. doi: 10.1016/j.neuroscience.2009.11.050. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
