Interstitial nephritis in melanoma patients secondary to PD-1 checkpoint inhibitor
- PMID: 28105370
- PMCID: PMC5240303
- DOI: 10.1186/s40425-016-0205-2
Interstitial nephritis in melanoma patients secondary to PD-1 checkpoint inhibitor
Abstract
Background: Immune checkpoint inhibitors have become the first line therapy in melanoma treatment and their use is extending to other malignancies. However, we are still learning about immune side effects produced by these drugs and their severity especially in patients with history of inflammatory diseases.
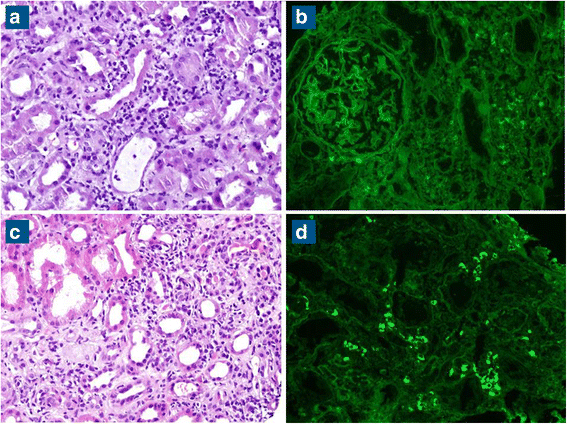
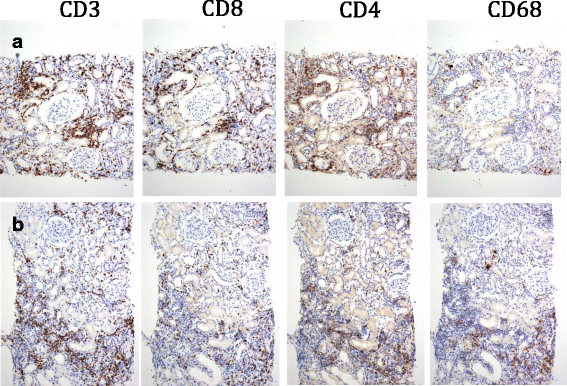
Case presentation: We present two cases of metastatic melanoma treated with nivolumab and pembrolizumab (anti PD-1). Both patients developed acute interstitial nephritis during immune checkpoint therapy. We emphasize the causal association between immune checkpoint inhibitors and the nephritis. The timing of drug administration and appearance of nephritis is suggestive of a causal relation between the checkpoint inhibitor therapy and this adverse event.
Conclusions: Although uncommon, some side effects from checkpoint inhibitors can be severe and may need to be addressed with immunosuppression. Given the increasing frequency of immunotherapy use, awareness should be raised in regards to immune side effects and their appropriate management.
Keywords: Immune checkpoint inhibitor; Interstitial nephritis; Nivolumab; PD-1 ligand (PD-L1); Pembrolizumab; Programed death 1 receptor (PD-1).
Figures
References
-
- Ribas A, Puzanov I, Dummer R, Schadendorf D, Hamid O, Robert C, et al. Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): a randomised, controlled, phase 2 trial. Lancet Oncol. 2015;16(8):908–918. doi: 10.1016/S1470-2045(15)00083-2. - DOI - PMC - PubMed
-
- Weber JS, D’Angelo SP, Minor D, Hodi FS, Gutzmer R, Neyns B, et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2015;16(4):375–384. doi: 10.1016/S1470-2045(15)70076-8. - DOI - PubMed
-
- Pharmaceutical company. Opdivo [package insert]. Princeton: Bristol-Myers Squibb Company; 2016.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
