First versus second year respiratory syncytial virus prophylaxis in chronic lung disease (2005-2015)
- PMID: 28105526
- PMCID: PMC5321716
- DOI: 10.1007/s00431-017-2849-4
First versus second year respiratory syncytial virus prophylaxis in chronic lung disease (2005-2015)
Abstract
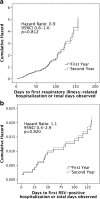
Children aged <2 years with chronic lung disease (CLD) have a 10-fold higher risk for respiratory syncytial virus-positive hospitalization (RSVH) compared to healthy term infants. Based on the updated position statements, we compared respiratory-related illness hospitalization (RIH) and RSVH risks in CLD children who received palivizumab during the first year (FY) versus second year (SY) of life in the Canadian Registry of Palivizumab (CARESS). Demographic data were collected at enrolment and RIH events recorded monthly from 2005 to 2015. Eight hundred forty-seven FY and 450 SY children with CLD were identified. SY children had a lower gestational age (27 versus 29 weeks) and required more days of respiratory support (64 versus 43), oxygen therapy (108 versus 55), and length of stay (118 versus 73) during the neonatal course compared to FY children; all p < 0.0005. RIH rates were 12.2 (FY) and 18.2 (SY), and RSVH rates were 2.3 (FY) and 3.9 (SY). Cox regression showed similar hazards for both RIH (hazard ratio 0.9, 95% CI 0.6-1.6, p = 0.812) and RSVH (hazard ratio 1.1, 95% CI 0.4-2.9, p = 0.920).
Conclusions: SY and FY children had similar risks for RIH and RSVH. The findings imply that SY children with CLD are correctly selected for palivizumab based on neonatal illness severity and merit prophylaxis. What is Known: • Children with chronic lung disease have a 10-fold higher risk for RSV-positive hospitalization in comparison to healthy term infants and commonly receive palivizumab prophylaxis as a preventative measure against serious RSV-related lower respiratory tract infections. • The American Academy of Pediatrics [ 2 ] and the Canadian Paediatric Society [ 30 ] have recently modified their recommendations for RSV prophylaxis in children with chronic lung disease, limiting palivizumab to either those <32 weeks gestation or those in the first year of life who are oxygen dependent or require medical therapy for the treatment of their condition. What is New: • Children with chronic lung disease receiving an additional course of palivizumab in their second year of life were determined to be at similar risk for both respiratory illness-related hospitalization and RSV-positive hospitalization as palivizumab-naïve children enrolled in the first year of life in the Canadian Registry for palivizumab (CARESS). • CARESS physicians are correctly identifying high-risk children with chronic lung disease in their second year of life, whom they believe will benefit from an additional year of palivizumab prophylaxis, based on neonatal illness severity.
Keywords: Chronic lung disease; Comparison; Outcomes; Palivizumab; Two seasons.
Conflict of interest statement
Conflict of interest
AbbVie had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. A.L., B.P., I.M., and K.L. have received investigator initiated research funding or received compensation as advisors or lecturers from AbbVie Corporation and MedImmune. D.W. has no conflicts of interest to declare. No honorarium, grant, or other form of payment was given to anyone to produce the manuscript.
Ethical approval
Research ethics board approval was obtained for the study at each participating site and all investigational procedures conform to the Declaration of Helsinki guiding principles.
Funding
The CARESS registry is funded by an investigator-initiated grant from AbbVie Corporation. The CARESS registry is registered under:
Informed consent
Informed consent is obtained from caregivers of all participating subjects prior to study enrollment. In the event of a hospitalization, relevant details are extracted from patient hospital records by the site’s research nurse following additional informed consent obtained from parents or legal guardians.
Figures
Similar articles
-
Respiratory Syncytial Virus Prophylaxis in Infants With Congenital Diaphragmatic Hernia in the Canadian Respiratory Syncytial Virus Evaluation Study of Palivizumab, 2005-2017.Clin Infect Dis. 2019 Aug 30;69(6):980-986. doi: 10.1093/cid/ciy1010. Clin Infect Dis. 2019. PMID: 30517603 Free PMC article.
-
Comparing First- and Second-year Palivizumab Prophylaxis in Patients With Hemodynamically Significant Congenital Heart Disease in the CARESS Database (2005-2015).Pediatr Infect Dis J. 2017 May;36(5):445-450. doi: 10.1097/INF.0000000000001357. Pediatr Infect Dis J. 2017. PMID: 28403044
-
Respiratory syncytial virus prophylaxis in infants with congenital airway anomalies compared to standard indications and complex medical disorders.Eur J Pediatr. 2019 Mar;178(3):377-385. doi: 10.1007/s00431-018-03308-1. Epub 2019 Jan 4. Eur J Pediatr. 2019. PMID: 30610419
-
Impact of the 2014 American Academy of Pediatrics recommendation and of the resulting limited financial coverage by the Italian Medicines Agency for palivizumab prophylaxis on the RSV-associated hospitalizations in preterm infants during the 2016-2017 epidemic season: a systematic review of seven Italian reports.Ital J Pediatr. 2019 Nov 9;45(1):139. doi: 10.1186/s13052-019-0736-5. Ital J Pediatr. 2019. PMID: 31706338 Free PMC article.
-
Respiratory syncytial virus prophylaxis for children with chronic lung disease: have we got the criteria right?Expert Rev Anti Infect Ther. 2019 Apr;17(4):211-222. doi: 10.1080/14787210.2019.1581062. Epub 2019 Feb 26. Expert Rev Anti Infect Ther. 2019. PMID: 30739513 Review.
Cited by
-
Prevalence and clinical characteristics of perinatal chronic lung disease by infant gestational age.J Neonatal Perinatal Med. 2021;14(1):43-51. doi: 10.3233/NPM-200412. J Neonatal Perinatal Med. 2021. PMID: 32474477 Free PMC article.
-
Effectiveness and Safety of Palivizumab for the Prevention of Serious Lower Respiratory Tract Infection Caused by Respiratory Syncytial Virus: A Systematic Review.Am J Perinatol. 2024 May;41(S 01):e1107-e1115. doi: 10.1055/a-1990-2633. Epub 2022 Nov 30. Am J Perinatol. 2024. PMID: 36452969 Free PMC article.
-
Respiratory syncytial virus hospitalization and incurred morbidities the season after prophylaxis.Paediatr Child Health. 2018 Nov;23(7):441-446. doi: 10.1093/pch/pxy046. Epub 2018 Apr 14. Paediatr Child Health. 2018. PMID: 30374219 Free PMC article.
-
Palivizumab for the Prophylaxis of Respiratory Syncytial Virus Disease: Expert Opinion and Recommendations for the Gulf Cooperation Council Region.Oman Med J. 2024 Sep 30;39(5):e667. doi: 10.5001/omj.2024.111. eCollection 2024 Sep. Oman Med J. 2024. PMID: 40248327 Free PMC article. Review.
-
Product review on the monoclonal antibody palivizumab for prevention of respiratory syncytial virus infection.Hum Vaccin Immunother. 2017 Sep 2;13(9):2138-2149. doi: 10.1080/21645515.2017.1337614. Epub 2017 Jun 12. Hum Vaccin Immunother. 2017. PMID: 28605249 Free PMC article. Review.
References
-
- American Academy of Pediatrics . Respiratory syncytial virus. In: Pickering LK, Baker CJ, Kimberlin DW, Long SS, editors. Red book: 2012 report of the Committee of Infectious Diseases. Illinois: Elk Grove Village; 2012. pp. 609–618.
-
- American Academy of Pediatrics Committee on Infectious Diseases (2014) Updated Guidance for Palivizumab Prophylaxis Among Infants and Young Children at Increased Risk of Hospitalization for Respiratory Syncytial Virus Infection. Pediatrics 134: 415-420 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

