Effects of cognitive behavioral therapy for insomnia and armodafinil on quality of life in cancer survivors: a randomized placebo-controlled trial
- PMID: 28105576
- PMCID: PMC5437869
- DOI: 10.1007/s11764-017-0597-0
Effects of cognitive behavioral therapy for insomnia and armodafinil on quality of life in cancer survivors: a randomized placebo-controlled trial
Abstract
Purpose: Cancer-related insomnia is associated with diminished quality of life (QOL), suggesting that improvement in insomnia may improve QOL in cancer survivors. Cognitive behavioral therapy for insomnia (CBT-I) has been shown to improve insomnia, but less is known regarding its effect on QOL and whether improvement in insomnia corresponds to improved QOL. The present analysis examines the effects of CBT-I, with and without armodafinil, on QOL both directly and indirectly through improvements of insomnia.
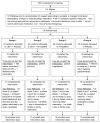
Methods: This is an analysis of 95 cancer survivors for a specified secondary aim of a four-arm randomized controlled trial assessing the combined and individual effects of CBT-I and armodafinil to improve insomnia. QOL and insomnia severity were assessed before, during the intervention, at post-intervention, and 3 months later by Functional Assessment of Cancer Therapy-General and Insomnia Severity Index, respectively.
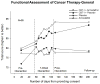
Results: Mean change in QOL from pre- to post-intervention for CBT-I + placebo, CBT-I + armodafinil, armodafinil, and placebo was 9.6 (SE = 1.8; p < 0.0001), 11.6 (SE = 1.8; p < 0.0001), -0.2 (SE = 3.2; p = 0.964), and 3.3 (SE = 2.0; p = 0.124), respectively. ANCOVA controlling for pre-intervention scores showed that participants receiving CBT-I had significantly improved QOL at post-intervention compared to those not receiving CBT-I (p < 0.0001, effect size = 0.57), with benefits being maintained at the 3-month follow-up. Path analysis revealed that this improvement in QOL was due to improvement in insomnia severity (p = 0.002), and Pearson correlations showed that changes in QOL from pre- to post-intervention were significantly associated with concurrent changes in insomnia severity (r = -0.56; p < 0.0001). Armodafinil had no effect on QOL for those who did or did not receive it (p = 0.976; effect size = -0.004).
Conclusion: In cancer survivors with insomnia, CBT-I resulted in clinically significant improvement in QOL via improvement in insomnia. This improvement in QOL remained stable even 3 months after completing CBT-I.
Implications for cancer survivors: Considering the high prevalence of insomnia and its detrimental impact on QOL in cancer survivors and the effectiveness of CBT-I in alleviating insomnia, it is important that evidence-based non-pharmacological sleep interventions such as CBT-I be provided as an integral part of cancer care.
Keywords: CBT-I; Cancer; Cancer survivors; Insomnia; Quality of life.
Conflict of interest statement
Conflict of interest
Dr. Perlis receives royalties for a variety of educational materials related to CBT-I; has received grant support for CBT-I related studies from Cephalon, Teva, and Sanofi-Aventis; has done consulting activities related to CBT-I from Lumosity, Sleep Easily, InsomniSolv, New York University, University of Buffalo and UCSD; and has received honoraria for multiple speaking engagements related to CBT-I. The remaining authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Figures

Similar articles
-
Cognitive behavioral therapy for insomnia, but not armodafinil, improves fatigue in cancer survivors with insomnia: a randomized placebo-controlled trial.Support Care Cancer. 2016 May;24(5):2059-2066. doi: 10.1007/s00520-015-2996-y. Epub 2015 Nov 5. Support Care Cancer. 2016. PMID: 26542272 Free PMC article. Clinical Trial.
-
Effects of armodafinil and cognitive behavior therapy for insomnia on sleep continuity and daytime sleepiness in cancer survivors.Sleep Med. 2016 Apr;20:18-24. doi: 10.1016/j.sleep.2015.12.010. Epub 2015 Dec 31. Sleep Med. 2016. PMID: 27318221 Free PMC article. Clinical Trial.
-
Randomized placebo-controlled trial of cognitive behavioral therapy and armodafinil for insomnia after cancer treatment.J Clin Oncol. 2015 Jan 10;33(2):165-71. doi: 10.1200/JCO.2014.57.6769. Epub 2014 Dec 1. J Clin Oncol. 2015. PMID: 25452447 Free PMC article. Clinical Trial.
-
Effects of cognitive-behavioral therapy for insomnia compared with controls among cancer survivors: a systematic review and meta-analysis of randomized trials.BMC Cancer. 2025 May 14;25(1):871. doi: 10.1186/s12885-025-14192-y. BMC Cancer. 2025. PMID: 40369457 Free PMC article.
-
Effects of Modafinil and Armodafinil in Patients With Obstructive Sleep Apnea: A Meta-analysis of Randomized Controlled Trials.Clin Ther. 2016 Apr;38(4):874-88. doi: 10.1016/j.clinthera.2016.02.004. Epub 2016 Feb 28. Clin Ther. 2016. PMID: 26923035
Cited by
-
The effect of a group cognitive behavioral therapy on the quality of life and emotional disturbance of women with breast cancer.Support Care Cancer. 2022 Jan;30(1):305-312. doi: 10.1007/s00520-021-06421-4. Epub 2021 Jul 19. Support Care Cancer. 2022. PMID: 34278530 Clinical Trial.
-
Behavioral Change Approaches to Sleep Disturbance in Cancer Patients: The History and the Road Ahead.Iran J Psychiatry. 2024 Oct;19(4):344-345. doi: 10.18502/ijps.v19i4.16547. Iran J Psychiatry. 2024. PMID: 40589833 Free PMC article. No abstract available.
-
Cancer-related fatigue and sleep deficiency in cancer care continuum: concepts, assessment, clusters, and management.Support Care Cancer. 2019 Jul;27(7):2747-2753. doi: 10.1007/s00520-019-04746-9. Epub 2019 Mar 22. Support Care Cancer. 2019. PMID: 30903367 Review.
-
Sleep and Cancer.Cancers (Basel). 2025 Mar 7;17(6):911. doi: 10.3390/cancers17060911. Cancers (Basel). 2025. PMID: 40149249 Free PMC article. Review.
-
Sleep Disorders in Cancer-A Systematic Review.Int J Environ Res Public Health. 2021 Nov 7;18(21):11696. doi: 10.3390/ijerph182111696. Int J Environ Res Public Health. 2021. PMID: 34770209 Free PMC article.
References
-
- American Cancer Society. Cancer Treatment & Survivorship Facts & Figures 2016–2017. Atlanta: American Cancer Society; 2016.
-
- Hewitt M, Rowland JH, Yancik R. Cancer survivors in the United States: age, health, and disability. The journals of gerontology Series A, Biological sciences and medical sciences. 2003;58(1):82–91. - PubMed
-
- Cella DF, Tulsky DS. Quality of life in cancer: definition, purpose, and method of measurement. Cancer Invest. 1993;11(3):327–36. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

