Nanopore Sensing
- PMID: 28105845
- PMCID: PMC5316487
- DOI: 10.1021/acs.analchem.6b04260
Nanopore Sensing
Figures


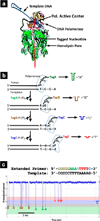

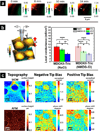




References
-
- Venkatesan BM, Bashir R. Nat. Nanotechnol. 2011;6:615–624. - PubMed
-
- Miles BN, Ivanov AP, Wilson KA, Dogan F, Japrung D, Edel JB. Chem. Soc. Rev. 2013;42:15–28. - PubMed
-
- Kasianowicz JJ, Robertson JW, Chan ER, Reiner JE, Stanford VM. Annu. Rev. Anal. Chem. 2008;1:737–766. - PubMed
-
- Gyurcsanyi RE. TrAC, Trends Anal. Chem. 2008;27:627–639.
-
- Friedman AK, Baker LA. In Nanoelectrochemistry. Boca Raton, FL: CRC Press; 2015. pp. 395–438.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

