Effect of Bone Marrow Aspirate Concentrate-Platelet-Rich Plasma on Tendon-Derived Stem Cells and Rotator Cuff Tendon Tear
- PMID: 28105983
- PMCID: PMC5657720
- DOI: 10.3727/096368917X694705
Effect of Bone Marrow Aspirate Concentrate-Platelet-Rich Plasma on Tendon-Derived Stem Cells and Rotator Cuff Tendon Tear
Abstract
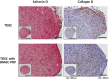
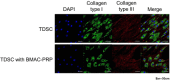
Bone marrow aspirate concentrates (BMACs) and platelet-rich plasma (PRP) are good sources to control the differentiation of tendon-derived stem cells (TDSCs), but there has been no study about the effect of the BMAC-PRP complex on TDSCs and tendinopathy. The aim of this study was to investigate the effect of BMAC-PRP on the TDSCs and to find the therapeutic effect of BMAC-PRP on the rotator cuff tendon tear. The chondrogenic and osteogenic potential of TDSCs decreased, but the adipogenic potential of TDSCs revealed no significant difference when they were cocultured with BMAC-PRP. Cell proliferation was significantly greater in TDSCs cocultured with BMAC-PRP than in TDSCs. The degree of wound closure (percentage) was different between TDSCs and TDSCs with BMAC-PRP. There was no significant difference in expression of collagen type I and type III in immunocytochemical staining in the presence of BMAC-PRP. Initial visual analog scale (VAS) score was 5.8 ± 1.9, which changed to 5.0 ± 2.3 at 3 weeks and 2.8 ± 2.3 at 3 months after the BMAC-PRP injection (p < 0.01). The American Shoulder Elbow Surgeon score changed from 39.4 ± 13.0 at baseline to 52.9 ± 22.9 at 3 weeks and 71.8 ± 19.7 at 3 months after the injection (p < 0.01). The initial torn area of the rotator cuff tendon was 30.2 ± 24.5 mm2, and this area was reduced to 22.5 ± 18.9 mm2 at 3 months, but the change was not significant (p > 0.05). The data indicate that BMAC-PRP enhances the proliferation and migration of TDSCs and prevents the aberrant chondrogenic and osteogenic differentiation of TDSCs, which might provide a mechanistic basis for the therapeutic benefits of BMAC-PRP for rotator cuff tendon tear.
Figures






Similar articles
-
Effects of bone marrow aspirate concentrate and platelet-rich plasma on patients with partial tear of the rotator cuff tendon.J Orthop Surg Res. 2018 Jan 3;13(1):1. doi: 10.1186/s13018-017-0693-x. J Orthop Surg Res. 2018. PMID: 29298726 Free PMC article. Clinical Trial.
-
Enhanced Tendon-to-Bone Healing of Chronic Rotator Cuff Tears by Bone Marrow Aspirate Concentrate in a Rabbit Model.Clin Orthop Surg. 2018 Mar;10(1):99-110. doi: 10.4055/cios.2018.10.1.99. Epub 2018 Feb 27. Clin Orthop Surg. 2018. PMID: 29564054 Free PMC article.
-
Bone Marrow Aspirate Concentrate Combined With an Appropriate Carrier Effectively Promotes Bone-Tendon Interface Healing in a Rabbit Model of Chronic Rotator Cuff Tear.Am J Sports Med. 2025 Mar;53(3):600-611. doi: 10.1177/03635465241313124. Epub 2025 Jan 28. Am J Sports Med. 2025. PMID: 39876035
-
A Comparison of Functional Outcomes in Rotator Cuff Repairs Using Adjunctive Bone Marrow Aspirate Concentrate vs. Bone Marrow Aspirate Concentrate With Platelet-Rich Plasma: A Systematic Review and Meta-Analysis.Cureus. 2024 Aug 23;16(8):e67594. doi: 10.7759/cureus.67594. eCollection 2024 Aug. Cureus. 2024. PMID: 39310448 Free PMC article. Review.
-
A New Tissue Engineering Strategy to Promote Tendon-bone Healing: Regulation of Osteogenic and Chondrogenic Differentiation of Tendon-derived Stem Cells.Orthop Surg. 2024 Oct;16(10):2311-2325. doi: 10.1111/os.14152. Epub 2024 Jul 23. Orthop Surg. 2024. PMID: 39043618 Free PMC article. Review.
Cited by
-
Ultrasonographic Findings in 41 Dogs Treated with Bone Marrow Aspirate Concentrate and Platelet-Rich Plasma for a Supraspinatus Tendinopathy: A Retrospective Study.Front Vet Sci. 2018 May 17;5:98. doi: 10.3389/fvets.2018.00098. eCollection 2018. Front Vet Sci. 2018. PMID: 29868619 Free PMC article.
-
Percutaneous bone marrow concentrate and platelet products versus exercise therapy for the treatment of rotator cuff tears: a randomized controlled, crossover trial with 2-year follow-up.BMC Musculoskelet Disord. 2024 May 18;25(1):392. doi: 10.1186/s12891-024-07519-6. BMC Musculoskelet Disord. 2024. PMID: 38762734 Free PMC article. Clinical Trial.
-
Dual-modal magnetic resonance and photoacoustic tracking and outcome of transplanted tendon stem cells in the rat rotator cuff injury model.Sci Rep. 2020 Aug 18;10(1):13954. doi: 10.1038/s41598-020-69214-5. Sci Rep. 2020. PMID: 32811841 Free PMC article.
-
Tendon stem/progenitor cell ageing: Modulation and rejuvenation.World J Stem Cells. 2019 Sep 26;11(9):677-692. doi: 10.4252/wjsc.v11.i9.677. World J Stem Cells. 2019. PMID: 31616543 Free PMC article. Review.
-
Wharton's Jelly-Derived Mesenchymal Stem Cells with High Aurora Kinase A Expression Show Improved Proliferation, Migration, and Therapeutic Potential.Stem Cells Int. 2022 Apr 11;2022:4711499. doi: 10.1155/2022/4711499. eCollection 2022. Stem Cells Int. 2022. PMID: 35450345 Free PMC article.
References
-
- Zhang G, Young BB, Ezura Y, Favata M, Soslowsky LJ, Chakravarti S, Birk DE. Development of tendon structure and function: Regulation of collagen fibrillogenesis. J Musculoskelet Neuronal Interact. 2005; 5: 5–21. - PubMed
-
- Wang JH. Mechanobiology of tendon. J Biomech. 2006; 39: 1563–82. - PubMed
-
- Sharma P, Maffulli N. Tendinopathy and tendon injury: The future. Disabil Rehabil. 2008; 30: 1733–15. - PubMed
-
- Kang JR, Gupta R. Mechanisms of fatty degeneration in massive rotator cuff tears. J Shoulder Elbow Surg. 2012; 21: 175–80. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

