Hepatic alterations are accompanied by changes to bile acid transporter-expressing neurons in the hypothalamus after traumatic brain injury
- PMID: 28106051
- PMCID: PMC5247752
- DOI: 10.1038/srep40112
Hepatic alterations are accompanied by changes to bile acid transporter-expressing neurons in the hypothalamus after traumatic brain injury
Abstract
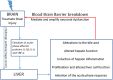
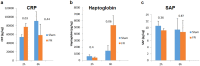
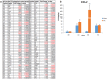
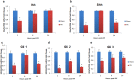
Annually, there are over 2 million incidents of traumatic brain injury (TBI) and treatment options are non-existent. While many TBI studies have focused on the brain, peripheral contributions involving the digestive and immune systems are emerging as factors involved in the various symptomology associated with TBI. We hypothesized that TBI would alter hepatic function, including bile acid system machinery in the liver and brain. The results show activation of the hepatic acute phase response by 2 hours after TBI, hepatic inflammation by 6 hours after TBI and a decrease in hepatic transcription factors, Gli 1, Gli 2, Gli 3 at 2 and 24 hrs after TBI. Bile acid receptors and transporters were decreased as early as 2 hrs after TBI until at least 24 hrs after TBI. Quantification of bile acid transporter, ASBT-expressing neurons in the hypothalamus, revealed a significant decrease following TBI. These results are the first to show such changes following a TBI, and are compatible with previous studies of the bile acid system in stroke models. The data support the emerging idea of a systemic influence to neurological disorders and point to the need for future studies to better define specific mechanisms of action.
Figures

Similar articles
-
Gene co-expression networks identify Trem2 and Tyrobp as major hubs in human APOE expressing mice following traumatic brain injury.Neurobiol Dis. 2017 Sep;105:1-14. doi: 10.1016/j.nbd.2017.05.006. Epub 2017 May 11. Neurobiol Dis. 2017. PMID: 28502803 Free PMC article.
-
Interfering with the Chronic Immune Response Rescues Chronic Degeneration After Traumatic Brain Injury.J Neurosci. 2016 Sep 21;36(38):9962-75. doi: 10.1523/JNEUROSCI.1898-15.2016. J Neurosci. 2016. PMID: 27656033 Free PMC article.
-
Increased miR-124-3p in microglial exosomes following traumatic brain injury inhibits neuronal inflammation and contributes to neurite outgrowth via their transfer into neurons.FASEB J. 2018 Jan;32(1):512-528. doi: 10.1096/fj.201700673R. Epub 2017 Sep 21. FASEB J. 2018. PMID: 28935818
-
Cellular players that shape evolving pathology and neurodegeneration following traumatic brain injury.Brain Behav Immun. 2018 Jul;71:9-17. doi: 10.1016/j.bbi.2018.03.033. Epub 2018 Mar 27. Brain Behav Immun. 2018. PMID: 29601944 Review.
-
Neurogenic inflammation after traumatic brain injury and its potentiation of classical inflammation.J Neuroinflammation. 2016 Oct 11;13(1):264. doi: 10.1186/s12974-016-0738-9. J Neuroinflammation. 2016. PMID: 27724914 Free PMC article. Review.
Cited by
-
Bile Acid Signaling Pathways from the Enterohepatic Circulation to the Central Nervous System.Front Neurosci. 2017 Nov 7;11:617. doi: 10.3389/fnins.2017.00617. eCollection 2017. Front Neurosci. 2017. PMID: 29163019 Free PMC article. Review.
-
Bile Acid Signaling in Neurodegenerative and Neurological Disorders.Int J Mol Sci. 2020 Aug 20;21(17):5982. doi: 10.3390/ijms21175982. Int J Mol Sci. 2020. PMID: 32825239 Free PMC article. Review.
-
Simultaneous Determination of Five Bile Acids as Potential Biomarkers for Alzheimer's Disease in Mouse Brain and Plasma.Anal Sci. 2021 Aug 10;37(8):1165-1170. doi: 10.2116/analsci.20P429. Epub 2021 Jan 29. Anal Sci. 2021. PMID: 33518588
-
Innate immune responses to trauma.Nat Immunol. 2018 Apr;19(4):327-341. doi: 10.1038/s41590-018-0064-8. Epub 2018 Mar 5. Nat Immunol. 2018. PMID: 29507356 Free PMC article. Review.
-
A Retrospective Clinical Analysis of the Serum Bile Acid Alteration Caused by Traumatic Brain Injury.Front Neurol. 2021 Aug 25;12:624378. doi: 10.3389/fneur.2021.624378. eCollection 2021. Front Neurol. 2021. PMID: 34512494 Free PMC article.
References
-
- Arvin B., Neville L. F., Barone F. C. & Feuerstein G. Z. Brain injury and inflammation. A putative role of TNF alpha. Ann N Y Acad Sci 765, 62–71; discussion 98-69 (1995). - PubMed
-
- Isaksson J., Lewen A., Hillered L. & Olsson Y. Up-regulation of intercellular adhesion molecule 1 in cerebral microvessels after cortical contusion trauma in a rat model. Acta Neuropathol 94, 16–20 (1997). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

