Phosphatidylinositol 3,4-bisphosphate regulates neurite initiation and dendrite morphogenesis via actin aggregation
- PMID: 28106075
- PMCID: PMC5339852
- DOI: 10.1038/cr.2017.13
Phosphatidylinositol 3,4-bisphosphate regulates neurite initiation and dendrite morphogenesis via actin aggregation
Abstract
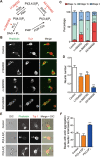
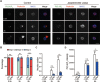
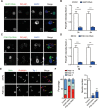
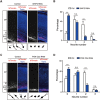
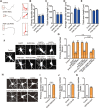
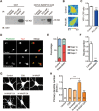
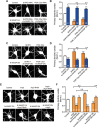
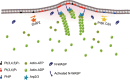
Neurite initiation is critical for neuronal morphogenesis and early neural circuit development. Recent studies showed that local actin aggregation underneath the cell membrane determined the site of neurite initiation. An immediately arising question is what signaling mechanism initiated actin aggregation. Here we demonstrate that local clustering of phosphatidylinositol 3,4-bisphosphate (PI(3,4)P2), a phospholipid with relatively few known signaling functions, is necessary and sufficient for aggregating actin and promoting neuritogenesis. In contrast, the related and more extensively studied phosphatidylinositol 4,5-bisphosphate or phosphatidylinositol (3,4,5)-trisphosphate (PIP3) molecules did not have such functions. Specifically, we showed that beads coated with PI(3,4)P2 promoted actin aggregation and neurite initiation, while pharmacological interference with PI(3,4)P2 synthesis inhibited both processes. PI(3,4)P2 clustering occurred even when actin aggregation was pharmacologically blocked, demonstrating that PI(3,4)P2 functioned as the upstream signaling molecule. Two enzymes critical for PI(3,4)P2 generation, namely, SH2 domain-containing inositol 5-phosphatase and class II phosphoinositide 3-kinase α, were complementarily and non-redundantly required for actin aggregation and neuritogenesis, as well as for subsequent dendritogenesis. Finally, we demonstrate that neural Wiskott-Aldrich syndrome protein and the Arp2/3 complex functioned downstream of PI(3,4)P2 to mediate neuritogenesis and dendritogenesis. Together, our results identify PI(3,4)P2 as an important signaling molecule during early development and demonstrate its critical role in regulating actin aggregation and neuritogenesis.
Figures


Similar articles
-
PI(3,5)P2 controls endosomal branched actin dynamics by regulating cortactin-actin interactions.J Cell Biol. 2015 Aug 31;210(5):753-69. doi: 10.1083/jcb.201412127. J Cell Biol. 2015. PMID: 26323691 Free PMC article.
-
Phosphatidylinositol (3,4) bisphosphate-specific phosphatases and effector proteins: A distinct branch of PI3K signaling.Cell Signal. 2015 Sep;27(9):1789-98. doi: 10.1016/j.cellsig.2015.05.013. Epub 2015 May 27. Cell Signal. 2015. PMID: 26022180 Review.
-
Actin Aggregations Mark the Sites of Neurite Initiation.Neurosci Bull. 2016 Feb;32(1):1-15. doi: 10.1007/s12264-016-0012-2. Epub 2016 Jan 18. Neurosci Bull. 2016. PMID: 26779918 Free PMC article.
-
Phosphoinositides and membrane curvature switch the mode of actin polymerization via selective recruitment of toca-1 and Snx9.Proc Natl Acad Sci U S A. 2013 Apr 30;110(18):7193-8. doi: 10.1073/pnas.1305286110. Epub 2013 Apr 15. Proc Natl Acad Sci U S A. 2013. PMID: 23589871 Free PMC article.
-
Regulation of podosomes by integrin alphavbeta3 and Rho GTPase-facilitated phosphoinositide signaling.Eur J Cell Biol. 2006 Apr;85(3-4):311-7. doi: 10.1016/j.ejcb.2006.01.008. Epub 2006 Feb 7. Eur J Cell Biol. 2006. PMID: 16460838 Review.
Cited by
-
Protein kinases: master regulators of neuritogenesis and therapeutic targets for axon regeneration.Cell Mol Life Sci. 2020 Apr;77(8):1511-1530. doi: 10.1007/s00018-019-03336-6. Epub 2019 Oct 28. Cell Mol Life Sci. 2020. PMID: 31659414 Free PMC article. Review.
-
In vitro reconstitution reveals phosphoinositides as cargo-release factors and activators of the ARF6 GAP ADAP1.Proc Natl Acad Sci U S A. 2021 Jan 5;118(1):e2010054118. doi: 10.1073/pnas.2010054118. Epub 2020 Dec 18. Proc Natl Acad Sci U S A. 2021. PMID: 33443153 Free PMC article.
-
Striking a balance: PIP2 and PIP3 signaling in neuronal health and disease.Explor Neuroprotective Ther. 2021;1:86-100. doi: 10.37349/ent.2021.00008. Epub 2021 Oct 29. Explor Neuroprotective Ther. 2021. PMID: 35098253 Free PMC article.
-
Class II PI3Ks at the Intersection between Signal Transduction and Membrane Trafficking.Biomolecules. 2019 Mar 15;9(3):104. doi: 10.3390/biom9030104. Biomolecules. 2019. PMID: 30884740 Free PMC article. Review.
-
An Efficient Screen for Cell-Intrinsic Factors Identifies the Chaperonin CCT and Multiple Conserved Mechanisms as Mediating Dendrite Morphogenesis.Front Cell Neurosci. 2020 Sep 25;14:577315. doi: 10.3389/fncel.2020.577315. eCollection 2020. Front Cell Neurosci. 2020. PMID: 33100975 Free PMC article.
References
-
- Craig AM, Banker G. Neuronal polarity. Annu Rev Neurosci 1994; 17:267–310. - PubMed
-
- Arimura N, Kaibuchi K. Neuronal polarity: from extracellular signals to intracellular mechanisms. Nat Rev Neurosci 2007; 8:194–205. - PubMed
-
- Cheng PL, Poo MM. Early events in axon/dendrite polarization. Annu Rev Neurosci 2012; 35:181–201. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

