Pharmacological characterisation of the highly NaV1.7 selective spider venom peptide Pn3a
- PMID: 28106092
- PMCID: PMC5247677
- DOI: 10.1038/srep40883
Pharmacological characterisation of the highly NaV1.7 selective spider venom peptide Pn3a
Erratum in
-
Corrigendum: Pharmacological characterisation of the highly NaV1.7 selective spider venom peptide Pn3a.Sci Rep. 2017 May 26;7:46816. doi: 10.1038/srep46816. Sci Rep. 2017. PMID: 28548111 Free PMC article. No abstract available.
Abstract
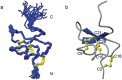
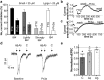
Human genetic studies have implicated the voltage-gated sodium channel NaV1.7 as a therapeutic target for the treatment of pain. A novel peptide, μ-theraphotoxin-Pn3a, isolated from venom of the tarantula Pamphobeteus nigricolor, potently inhibits NaV1.7 (IC50 0.9 nM) with at least 40-1000-fold selectivity over all other NaV subtypes. Despite on-target activity in small-diameter dorsal root ganglia, spinal slices, and in a mouse model of pain induced by NaV1.7 activation, Pn3a alone displayed no analgesic activity in formalin-, carrageenan- or FCA-induced pain in rodents when administered systemically. A broad lack of analgesic activity was also found for the selective NaV1.7 inhibitors PF-04856264 and phlotoxin 1. However, when administered with subtherapeutic doses of opioids or the enkephalinase inhibitor thiorphan, these subtype-selective NaV1.7 inhibitors produced profound analgesia. Our results suggest that in these inflammatory models, acute administration of peripherally restricted NaV1.7 inhibitors can only produce analgesia when administered in combination with an opioid.
Figures






References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

