Effect of myocyte-fibroblast coupling on the onset of pathological dynamics in a model of ventricular tissue
- PMID: 28106124
- PMCID: PMC5247688
- DOI: 10.1038/srep40985
Effect of myocyte-fibroblast coupling on the onset of pathological dynamics in a model of ventricular tissue
Abstract
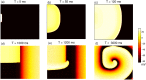
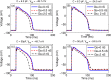
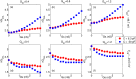
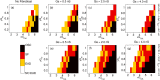
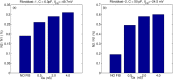
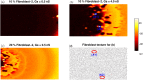
Managing lethal cardiac arrhythmias is one of the biggest challenges in modern cardiology, and hence it is very important to understand the factors underlying such arrhythmias. While early afterdepolarizations (EAD) of cardiac cells is known to be one such arrhythmogenic factor, the mechanisms underlying the emergence of tissue level arrhythmias from cellular level EADs is not fully understood. Another known arrhythmogenic condition is fibrosis of cardiac tissue that occurs both due to aging and in many types of heart diseases. In this paper we describe the results of a systematic in-silico study, using the TNNP model of human cardiac cells and MacCannell model for (myo)fibroblasts, on the possible effects of diffuse fibrosis on arrhythmias occurring via EADs. We find that depending on the resting potential of fibroblasts (VFR), M-F coupling can either increase or decrease the region of parameters showing EADs. Fibrosis increases the probability of occurrence of arrhythmias after a single focal stimulation and this effect increases with the strength of the M-F coupling. While in our simulations, arrhythmias occur due to fibrosis induced ectopic activity, we do not observe any specific fibrotic pattern that promotes the occurrence of these ectopic sources.
Figures


Similar articles
-
Arrhythmogenic consequences of myofibroblast-myocyte coupling.Cardiovasc Res. 2012 Feb 1;93(2):242-51. doi: 10.1093/cvr/cvr292. Epub 2011 Nov 2. Cardiovasc Res. 2012. PMID: 22049532 Free PMC article.
-
A study of early afterdepolarizations in a model for human ventricular tissue.PLoS One. 2014 Jan 10;9(1):e84595. doi: 10.1371/journal.pone.0084595. eCollection 2014. PLoS One. 2014. PMID: 24427289 Free PMC article.
-
Slow [Na]i Changes and Positive Feedback Between Membrane Potential and [Ca]i Underlie Intermittent Early Afterdepolarizations and Arrhythmias.Circ Arrhythm Electrophysiol. 2015 Dec;8(6):1472-80. doi: 10.1161/CIRCEP.115.003085. Epub 2015 Sep 25. Circ Arrhythm Electrophysiol. 2015. PMID: 26407967 Free PMC article.
-
Cardiac fibrosis and arrhythmogenesis: the road to repair is paved with perils.J Mol Cell Cardiol. 2014 May;70:83-91. doi: 10.1016/j.yjmcc.2013.10.018. Epub 2013 Oct 31. J Mol Cell Cardiol. 2014. PMID: 24184999 Free PMC article. Review.
-
Arrhythmogenic implications of fibroblast-myocyte interactions.Circ Arrhythm Electrophysiol. 2012 Apr;5(2):442-52. doi: 10.1161/CIRCEP.110.957647. Circ Arrhythm Electrophysiol. 2012. PMID: 22511661 Review. No abstract available.
Cited by
-
Modeling the human heart ex vivo-current possibilities and strive for future applications.J Tissue Eng Regen Med. 2022 Oct;16(10):853-874. doi: 10.1002/term.3335. Epub 2022 Jun 24. J Tissue Eng Regen Med. 2022. PMID: 35748158 Free PMC article. Review.
-
Editorial: Interplay between the heart and the immune system: Focus on heart rhythm regulation.Front Physiol. 2022 Aug 10;13:981499. doi: 10.3389/fphys.2022.981499. eCollection 2022. Front Physiol. 2022. PMID: 36035479 Free PMC article. No abstract available.
-
Heterogeneous Effects of Fibroblast-Myocyte Coupling in Different Regions of the Human Atria Under Conditions of Atrial Fibrillation.Front Physiol. 2019 Jul 4;10:847. doi: 10.3389/fphys.2019.00847. eCollection 2019. Front Physiol. 2019. PMID: 31333496 Free PMC article.
-
Mechanistic investigation of Ca2+ alternans in human heart failure and its modulation by fibroblasts.PLoS One. 2019 Jun 18;14(6):e0217993. doi: 10.1371/journal.pone.0217993. eCollection 2019. PLoS One. 2019. PMID: 31211790 Free PMC article.
-
Are Interactions between Epicardial Adipose Tissue, Cardiac Fibroblasts and Cardiac Myocytes Instrumental in Atrial Fibrosis and Atrial Fibrillation?Cells. 2021 Sep 21;10(9):2501. doi: 10.3390/cells10092501. Cells. 2021. PMID: 34572150 Free PMC article. Review.
References
-
- Sinha S. & Sridhar S. Patterns in Excitable Media: Genesis, Dynamics, and Control (CRC Press, 2014).
-
- Keener J. P. & Sneyd J. Mathematical physiology, vol. 1 (Springer, 1998).
-
- Heron M. Deaths: leading causes for 2010. National vital statistics reports: from the Centers for Disease Control and Prevention, National Center for Health Statistics, National Vital Statistics System 62(6), 1–96 (2013). - PubMed
-
- Sridhar S., Sinha S. & Panfilov A. V. Anomalous drift of spiral waves in heterogeneous excitable media. Physical Review E 82(5), 051908 (2010). - PubMed
-
- Sridhar S., Ghosh A. & Sinha S. Critical role of pinning defects in scroll-wave breakup in active media. EPL (Europhysics Letters) 103(5), 50003 (2013).
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

