Identification of LukPQ, a novel, equid-adapted leukocidin of Staphylococcus aureus
- PMID: 28106142
- PMCID: PMC5247767
- DOI: 10.1038/srep40660
Identification of LukPQ, a novel, equid-adapted leukocidin of Staphylococcus aureus
Abstract
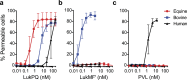
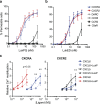
Bicomponent pore-forming leukocidins are a family of potent toxins secreted by Staphylococcus aureus, which target white blood cells preferentially and consist of an S- and an F-component. The S-component recognizes a receptor on the host cell, enabling high-affinity binding to the cell surface, after which the toxins form a pore that penetrates the cell lipid bilayer. Until now, six different leukocidins have been described, some of which are host and cell specific. Here, we identify and characterise a novel S. aureus leukocidin; LukPQ. LukPQ is encoded on a 45 kb prophage (ΦSaeq1) found in six different clonal lineages, almost exclusively in strains cultured from equids. We show that LukPQ is a potent and specific killer of equine neutrophils and identify equine-CXCRA and CXCR2 as its target receptors. Although the S-component (LukP) is highly similar to the S-component of LukED, the species specificity of LukPQ and LukED differs. By forming non-canonical toxin pairs, we identify that the F-component contributes to the observed host tropism of LukPQ, thereby challenging the current paradigm that leukocidin specificity is driven solely by the S-component.
Figures



References
-
- Viana D. et al. Adaptation of Staphylococcus aureus to ruminant and equine hosts involves SaPI-carried variants of von Willebrand factor-binding protein. Mol. Microbiol. 77, 1583–1594 (2010). - PubMed
-
- McCarthy A. J. & Lindsay J. A. Staphylococcus aureus innate immune evasion is lineage-specific: A bioinfomatics study. Infect. Genet. Evol. 19, 7–14 (2013). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- HICF-T5-342/DH_/Department of Health/United Kingdom
- G1001787/MRC_/Medical Research Council/United Kingdom
- WT098600/WT_/Wellcome Trust/United Kingdom
- BB/I013873/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/K00638X/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources

