Primidone inhibits TRPM3 and attenuates thermal nociception in vivo
- PMID: 28106668
- PMCID: PMC5402713
- DOI: 10.1097/j.pain.0000000000000846
Primidone inhibits TRPM3 and attenuates thermal nociception in vivo
Abstract
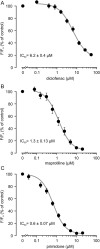
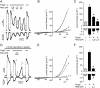
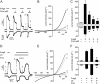
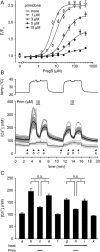
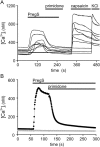
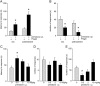
The melastatin-related transient receptor potential (TRP) channel TRPM3 is a nonselective cation channel expressed in nociceptive neurons and activated by heat. Because TRPM3-deficient mice show inflammatory thermal hyperalgesia, pharmacological inhibition of TRPM3 may exert antinociceptive properties. Fluorometric Ca influx assays and a compound library containing approved or clinically tested drugs were used to identify TRPM3 inhibitors. Biophysical properties of channel inhibition were assessed using electrophysiological methods. The nonsteroidal anti-inflammatory drug diclofenac, the tetracyclic antidepressant maprotiline, and the anticonvulsant primidone were identified as highly efficient TRPM3 blockers with half-maximal inhibition at 0.6 to 6 μM and marked specificity for TRPM3. Most prominently, primidone was biologically active to suppress TRPM3 activation by pregnenolone sulfate (PregS) and heat at concentrations markedly lower than plasma concentrations commonly used in antiepileptic therapy. Primidone blocked PregS-induced Cai influx through TRPM3 by allosteric modulation and reversibly inhibited atypical inwardly rectifying TRPM3 currents induced by coapplication of PregS and clotrimazole. In vivo, analgesic effects of low doses of primidone were demonstrated in mice, applying PregS- and heat-induced pain models, including inflammatory hyperalgesia. Thus, applying the approved drug at concentrations that are lower than those needed to induce anticonvulsive effects offers a shortcut for studying physiological and pathophysiological roles of TRPM3 in vivo.
Conflict of interest statement
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Figures



References
-
- Alvin J, Goh E, Bush MT. Study of the hepatic metabolism of primidone by improved methodology. J Pharmacol Exp Ther 1975;194:117–25. - PubMed
-
- Bootman MD, Rietdorf K, Collins T, Walker S, Sanderson M. Ca2+-Sensitive fluorescent dyes and intracellular Ca2+ imaging. In: Parys JB, Bootman M, Yule DI, Bultynck G, editors. Calcium techniques. Cold Spring Habor: A Laboratory Manual Cold Spring Habor Laboratory Press, 2014. p. 25–41.
-
- Budakova L, Brozmanova H, Grundmann M, Fischer J. Simultaneous determination of antiepileptic drugs and their two active metabolites by HPLC. J Sep Sci 2008;31:1–8. - PubMed
-
- Carmody J, Knodler L, Murray S. Paradoxical modulation of nociception in mice by barbiturate agonism and antagonism: is a GABA site involved in nociception? Eur J Neurosci 1991;3:833–8. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

