Combination of Morroniside and Diosgenin Prevents High Glucose-Induced Cardiomyocytes Apoptosis
- PMID: 28106847
- PMCID: PMC6155861
- DOI: 10.3390/molecules22010163
Combination of Morroniside and Diosgenin Prevents High Glucose-Induced Cardiomyocytes Apoptosis
Abstract
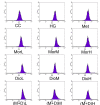
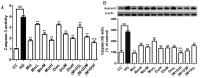
Cornus officinalis and Dioscorea opposita are two traditional Chinese medicines widely used in China for treating diabetes mellitus and its complications, such as diabetic cardiomyopathy. Morroniside (Mor) of Cornus officinalis and diosgenin (Dio) of Dioscorea opposita formed an innovative formula named M + D. The aims of the present study were to investigate myocardial protective effect of M + D on diabetic cardiomyopathy (DCM) through the inhibition of expression levels of caspase-3 protein, and identify the advantage of M + D compared with Mor, Dio, and the positive drug metformin (Met). We detected cell viability, cell apoptosis, intracellular reactive oxygen species (ROS) levels, and the expression levels of Bcl-2, Bax, and caspase-3 protein in rat cardiomyocytes. In result, Mor, Dio, and M + D increased cell viability, inhibited cell apoptosis and decreased ROS levels. Additionally, the expression of Bax and Bcl-2 protein was modulated and the expression levels of caspase-3 protein were markedly decreased. Among the treatment groups, M + D produced the most prominent effects. In conclusion, our data showed for the first time that Mor, Dio, and M + D prevented high glucose (HG)-induced myocardial injury by reducing oxidative stress and apoptosis in rat cardiomyocytes. Among all the groups, M + D produced the strongest effect, while Mor and Dio produced weaker effects.
Keywords: Bcl-2/Bax/caspase-3 signaling pathway; cardiomyocytes; diabetic cardiomyopathy; diosgenin; morroniside.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Chen Y., Wu Y., Gan X., Liu K., Xing L.V., Shen H., Dai G., Xu H. Iridoid glycoside from Cornus officinalis ameliorated diabetes mellitus-induced testicular damage in male rats: Involvement of suppression of the AGEs/RAGE/p38 MAPK signaling pathway. J. Ethnopharmacol. 2016;194:850–860. doi: 10.1016/j.jep.2016.10.079. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

