Report of 12-months efficacy and safety of intravitreal fluocinolone acetonide implant for the treatment of chronic diabetic macular oedema: a real-world result in the United Kingdom
- PMID: 28106887
- PMCID: PMC5396008
- DOI: 10.1038/eye.2016.301
Report of 12-months efficacy and safety of intravitreal fluocinolone acetonide implant for the treatment of chronic diabetic macular oedema: a real-world result in the United Kingdom
Abstract
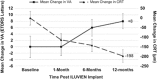
PurposeTo report the 12-months visual and anatomical outcomes of chronic diabetic macular oedema (DMO) treated with ILUVIEN in a real-world clinical practice in a single tertiary referral centre.MethodRetrospective data collection and analysis of consecutive 28 eyes of 23 diabetic patients received ILUVIEN implant for refractory DMO. Standard assessment included visual acuity (VA), central retinal thickness (CRT), slit-lamp biomicroscopy, and Goldmann tonometry for intraocular pressure (IOP) at 1, 6, and 12 months.ResultsBaseline mean VA was 47 (SD 18) letters improved to 55 (SD 17) letters (P=0.004) at 12 months. VA was improved in 16 eyes (57%), stabilised in 9 eyes (32%), and decreased in 3 eyes (11%). Seven eyes (25%) gained ≥15 letters, and 10 eyes (36%) gained >10 letters from baseline. The percentage of eyes achieved driving vision (≥70 Early Treatment Diabetic Retinopathy Study letters) was doubled from baseline 18 to 36% at 6 months and 32% at 12 months. Mean CRT decreased by 198 μm from baseline 494 μm (SD 191) to 296 μm (SD 121) at 12 months (P<0.001). Two eyes received additional anti-vascular endothelial growth factor injections after 10 months.
Complications: Raised IOP in three eyes (11%) controlled with IOP-lowering drops, vitreous haemorrhage in one eye and one endophthalmitis (1 year vision improved to 6/24).ConclusionOur real-world results show that the visual and the anatomical improvements achieved by a single ILUVIEN implant injection were maintained up to 12 months with minimal adjunctive therapy. IOP monitoring remains essential in ILUVIEN patients, although our study shows a relatively low risk of IOP elevation post ILUVIEN injection, even in existing controlled ocular hypertension. Our results demonstrate that ILUVIEN is an effective long-term option in treating chronic refractory DMO.
Conflict of interest statement
FA, PLL and RC declare no conflict of interest. SE is an Advisory Board Member of and speaker for Alimera Science, Bayer, Novartis, and Alcon. AM is an Advisory Board Member of Alimera Sciences. BM is an Advisory Board Member of and speaker for Alimera Science, Allergan, Bayer, Novartis, and ORAYA therapeutics.
Figures
References
-
- Moss SE, Klein R, Klein BE. Ten-year incidence of visual loss in a diabetic population. Ophthalmology 1994; 101: 1061–1070. - PubMed
-
- Antonetti DA, Lieth E, Barber AJ, Gardner TW. Molecular mechanisms of vascular permeability in diabetic retinopathy. Semin Ophthalmol 1999; 14: 240–248. - PubMed
-
- Klaassen I, Van Noorden CJ, Schlingemann RO. Molecular basis of the inner blood–retinal barrier and its breakdown in diabetic macular edema and other pathological conditions. Prog Retin Eye Res 2013; 34: 19–48. - PubMed
-
- Ehrlich R, Harris A, Ciulla TA, Kheradiya N, Winston DM, Wirostko B. Diabetic macular oedema: physical, physiological and molecular factors contribute to this pathological process. Acta Ophthalmol 2010; 88: 279–291. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials