The 2016 Bowman Lecture Conjunctival curses: scarring conjunctivitis 30 years on
- PMID: 28106896
- PMCID: PMC5306467
- DOI: 10.1038/eye.2016.284
The 2016 Bowman Lecture Conjunctival curses: scarring conjunctivitis 30 years on
Abstract
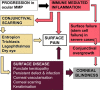
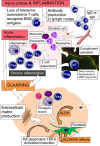
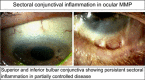
This review is in two sections. The first section summarises 35 conditions, both common and infrequent, causing cicatrising conjunctivitis. Guidelines for making a diagnosis are given together with the use of diagnostic tests, including direct and indirect immunofluorescence, and their interpretation. The second section evaluates our knowledge of ocular mucous membrane pemphigoid, which is the commonest cause of cicatrizing conjunctivitis in most developed countries. The clinical characteristics, demographics, and clinical signs of the disease are described. This is followed by a review and re-evaluation of the pathogenesis of conjunctival inflammation in mucous membrane pemphigoid (MMP), resulting in a revised hypothesis of the autoimmune mechanisms causing inflammation in ocular MMP. The relationship between inflammation and scarring in MMP conjunctiva is described. Recent research, describing the role of aldehyde dehydrogenase (ALDH) and retinoic acid (RA) in both the initiation and perpetuation of profibrotic activity in MMP conjunctival fibroblasts is summarised and the potential for antifibrotic therapy, using ALDH inhibition, is discussed. The importance of the management of the ocular surface in MMP is briefly summarised. This is followed with the rationale for the use of systemic immunomodulatory therapy, currently the standard of care for patients with active ocular MMP. The evidence for the use of these drugs is summarised and guidelines given for their use. Finally, the areas for research and innovation in the next decade are reviewed including the need for better diagnostics, markers of disease activity, and the potential for biological and topical therapies for both inflammation and scarring.
Conflict of interest statement
JK Dart is involved with the development of ALDH inhibition for scarring under the Patent PCT/GB2015/051292 held by UCL Business plc.
Figures

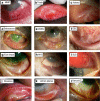


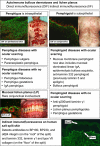

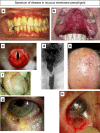
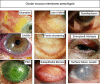

References
-
- Power H. Prefatory Memoir. In Burdon-Sanderson J, Hulke JW (eds The Collected Papers of Sir William Bowman, Bart., F.R.S.. Vol. 2 Harrison and Sons: London, 1892. pp xi–xxiii.
-
- Thorne JE, Anhalt GJ, Jabs DA. Mucous membrane pemphigoid and pseudopemphigoid. Ophthalmology 2004; 111: 45–52. - PubMed
-
- Guglielmetti S, Dart JK, Calder V. Atopic keratoconjunctivitis and atopic dermatitis. Curr Opin Allergy Clin Immunol 2010; 10: 478–485. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

