Effects of Infant Formula With Human Milk Oligosaccharides on Growth and Morbidity: A Randomized Multicenter Trial
- PMID: 28107288
- PMCID: PMC5378003
- DOI: 10.1097/MPG.0000000000001520
Effects of Infant Formula With Human Milk Oligosaccharides on Growth and Morbidity: A Randomized Multicenter Trial
Abstract
Objectives: The aim of the study was to evaluate the effects of infant formula supplemented with 2 human milk oligosaccharides (HMOs) on infant growth, tolerance, and morbidity.
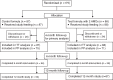
Methods: Healthy infants, 0 to 14 days old, were randomized to an intact-protein, cow's milk-based infant formula (control, n = 87) or the same formula with 1.0 g/L 2'fucosyllactose (2'FL) and 0.5 g/L lacto-N-neotetraose (LNnT) (test, n = 88) from enrollment to 6 months; all infants received standard follow-up formula without HMOs from 6 to 12 months. Primary endpoint was weight gain through 4 months. Secondary endpoints included additional anthropometric measures, gastrointestinal tolerance, behavioral patterns, and morbidity through age 12 months.
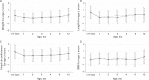
Results: Weight gain was similar in both groups (mean difference [95% confidence interval] test vs control: -0.30 [-1.94, 1.34] g/day; lower bound of 95% confidence interval was above noninferiority margin [-3 g/day]). Digestive symptoms and behavioral patterns were similar between groups; exceptions included softer stool (P = 0.021) and fewer nighttime wake-ups (P = 0.036) in the test group at 2 months. Infants receiving test (vs control) had significantly fewer parental reports (P = 0.004-0.047) of bronchitis through 4 (2.3% vs 12.6%), 6 (6.8% vs 21.8%), and 12 months (10.2% vs 27.6%); lower respiratory tract infection (adverse event cluster) through 12 months (19.3% vs 34.5%); antipyretics use through 4 months (15.9% vs 29.9%); and antibiotics use through 6 (34.1% vs 49.4%) and 12 months (42.0% vs 60.9%).
Conclusions: Infant formula with 2'FL and LNnT is safe, well-tolerated, and supports age-appropriate growth. Secondary outcome findings showing associations between consuming HMO-supplemented formula and lower parent-reported morbidity (particularly bronchitis) and medication use (antipyretics and antibiotics) warrant confirmation in future studies.
Trial registration: ClinicalTrials.gov NCT01715246.
Figures

References
-
- Coppa GV, Zampini L, Galeazzi T, et al. Prebiotics in human milk: a review. Dig Liver Dis 2006; 38 suppl 2:S291–S294. - PubMed
-
- Coppa GV, Pierani P, Zampini L, et al. Oligosaccharides in human milk during different phases of lactation. Acta Paediatr Suppl 1999; 88:89–94. - PubMed
-
- Hamosh M. Bioactive factors in human milk. Pediatr Clin North Am 2001; 48:69–86. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

