Complex Dynamics of Virus Spread from Low Infection Multiplicities: Implications for the Spread of Oncolytic Viruses
- PMID: 28107341
- PMCID: PMC5249046
- DOI: 10.1371/journal.pcbi.1005241
Complex Dynamics of Virus Spread from Low Infection Multiplicities: Implications for the Spread of Oncolytic Viruses
Erratum in
-
Correction: Complex Dynamics of Virus Spread from Low Infection Multiplicities: Implications for the Spread of Oncolytic Viruses.PLoS Comput Biol. 2017 May 19;13(5):e1005548. doi: 10.1371/journal.pcbi.1005548. eCollection 2017 May. PLoS Comput Biol. 2017. PMID: 28542168 Free PMC article.
Abstract
While virus growth dynamics have been well-characterized in several infections, data are typically collected once the virus population becomes easily detectable. Earlier dynamics, however, remain less understood. We recently reported unusual early dynamics in an experimental system using adenovirus infection of human embryonic kidney (293) cells. Under identical experimental conditions, inoculation at low infection multiplicities resulted in either robust spread, or in limited spread that eventually stalled, with both outcomes occurring with approximately equal frequencies. The reasons underlying these observations have not been understood. Here, we present further experimental data showing that inhibition of interferon-induced antiviral states in cells results in a significant increase in the percentage of robust infections that are observed, implicating a race between virus replication and the spread of the anti-viral state as a central mechanism. Analysis of a variety of computational models, however, reveals that this alone cannot explain the simultaneous occurrence of both viral growth outcomes under identical conditions, and that additional biological mechanisms have to be invoked to explain the data. One such mechanism is the ability of the virus to overcome the antiviral state through multiple infection of cells. If this is included in the model, two outcomes of viral spread are found to be simultaneously stable, depending on initial conditions. In stochastic versions of such models, the system can go by chance to either state from identical initial conditions, with the relative frequency of the outcomes depending on the strength of the interferon-based anti-viral response, consistent with the experiments. This demonstrates considerable complexity during the early phase of the infection that can influence the ability of a virus to become successfully established. Implications for the initial dynamics of oncolytic virus spread through tumors are discussed.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
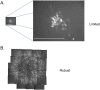
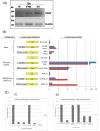
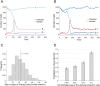
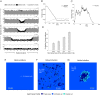
References
-
- Nowak MA, May RM (2000) Virus dynamics. Mathematical principles of immunology and virology.: Oxford University Press.
-
- Perelson AS (2002) Modelling viral and immune system dynamics. Nature Rev Immunol 2: 28–36. - PubMed
-
- Bangham CRM, Hall S.E., Jeffrey K.J.M, Vine A.M., Witkover A., Nowak M.A., Wodarz D., Usuku K., Osame M. (1999) Genetic control and dynamics of the cellular immune response to the human T-cell leukaemia virus, HTLV-1. Philosophical Transactions of the Royal Society, Series B 354: 691–700. - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

