Syk Regulates Neutrophilic Airway Hyper-Responsiveness in a Chronic Mouse Model of Allergic Airways Inflammation
- PMID: 28107345
- PMCID: PMC5249072
- DOI: 10.1371/journal.pone.0163614
Syk Regulates Neutrophilic Airway Hyper-Responsiveness in a Chronic Mouse Model of Allergic Airways Inflammation
Abstract
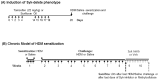
Background: Asthma is a chronic inflammatory disease characterized by airways hyper-responsiveness (AHR), reversible airway obstruction, and airway inflammation and remodeling. We previously showed that Syk modulates methacholine-induced airways contractility in naïve mice and in mice with allergic airways inflammation. We hypothesize that Syk plays a role in the pathogenesis of AHR; this was evaluated in a chronic 8-week mouse model of house dust mite (HDM)-induced allergic airways inflammation.
Methods: We used the Sykflox/flox//rosa26CreERT2 conditional Syk knock-out mice to assess the role of Syk prior to HDM exposure, and treated HDM-sensitized mice with the Syk inhibitor, GSK143, to evaluate its role in established allergic airways inflammation. Respiratory mechanics and methacholine (MCh)-responsiveness were assessed using the flexiVent® system. Lungs underwent bronchoalveolar lavage to isolate inflammatory cells or were frozen for determination of gene expression in tissues.
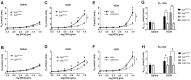
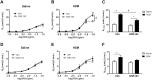
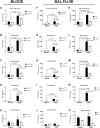
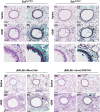
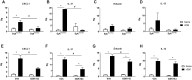
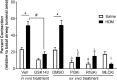
Results: MCh-induced AHR was observed following HDM sensitization in the Syk-intact (Sykflox/flox) and vehicle-treated BALB/c mice. MCh responsiveness was reduced to control levels in HDM-sensitized Sykdel/del mice and in BALB/c and Sykflox/flox mice treated with GSK143. Both Sykdel/del and GSK143-treated mice mounted appropriate immune responses to HDM, with HDM-specific IgE levels that were comparable to Sykflox/flox and vehicle-treated BALB/c mice. HDM-induced increases in bronchoalveolar lavage cell counts were attenuated in both Sykdel/del and GSK143-treated mice, due primarily to decreased neutrophil recruitment. Gene expression analysis of lung tissues revealed that HDM-induced expression of IL-17 and CXCL-1 was significantly attenuated in both Sykdel/del and GSK143-treated mice.
Conclusion: Syk inhibitors may play a role in the management of neutrophilic asthma.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


Similar articles
-
A 4-Week Model of House Dust Mite (HDM) Induced Allergic Airways Inflammation with Airway Remodeling.Sci Rep. 2018 May 2;8(1):6925. doi: 10.1038/s41598-018-24574-x. Sci Rep. 2018. PMID: 29720689 Free PMC article.
-
Spleen tyrosine kinase inhibition attenuates airway hyperresponsiveness and pollution-induced enhanced airway response in a chronic mouse model of asthma.J Allergy Clin Immunol. 2013 Feb;131(2):512-20.e1-10. doi: 10.1016/j.jaci.2012.07.039. Epub 2012 Sep 13. J Allergy Clin Immunol. 2013. PMID: 22981792
-
Syk mediates airway contractility independent of leukocyte function.Allergy. 2015 Apr;70(4):429-35. doi: 10.1111/all.12564. Epub 2015 Jan 26. Allergy. 2015. PMID: 25556883
-
The interleukin-33 receptor ST2 is important for the development of peripheral airway hyperresponsiveness and inflammation in a house dust mite mouse model of asthma.Clin Exp Allergy. 2016 Mar;46(3):479-90. doi: 10.1111/cea.12683. Clin Exp Allergy. 2016. PMID: 26609909
-
Dietary galacto-oligosaccharides prevent airway eosinophilia and hyperresponsiveness in a murine house dust mite-induced asthma model.Respir Res. 2015 Feb 7;16(1):17. doi: 10.1186/s12931-015-0171-0. Respir Res. 2015. PMID: 25849971 Free PMC article.
Cited by
-
A 4-Week Model of House Dust Mite (HDM) Induced Allergic Airways Inflammation with Airway Remodeling.Sci Rep. 2018 May 2;8(1):6925. doi: 10.1038/s41598-018-24574-x. Sci Rep. 2018. PMID: 29720689 Free PMC article.
-
A Synthetic Cross-Species CD200R1 Agonist Suppresses Inflammatory Immune Responses In Vivo.Mol Ther Nucleic Acids. 2018 Sep 7;12:350-358. doi: 10.1016/j.omtn.2018.05.023. Epub 2018 Jul 3. Mol Ther Nucleic Acids. 2018. PMID: 30195773 Free PMC article.
-
LC3-Associated Phagocytosis Is Required for Dendritic Cell Inflammatory Cytokine Response to Gut Commensal Yeast Saccharomyces cerevisiae.Front Immunol. 2017 Oct 25;8:1397. doi: 10.3389/fimmu.2017.01397. eCollection 2017. Front Immunol. 2017. PMID: 29118762 Free PMC article.
-
Assessing the Anti-Inflammatory Effects of an Orally Dosed Enzymatically Liberated Fish Oil in a House Dust Model of Allergic Asthma.Biomedicines. 2022 Oct 14;10(10):2574. doi: 10.3390/biomedicines10102574. Biomedicines. 2022. PMID: 36289834 Free PMC article.
-
Spleen tyrosine kinase facilitates neutrophil activation and worsens long-term neurologic deficits after spinal cord injury.J Neuroinflammation. 2021 Dec 24;18(1):302. doi: 10.1186/s12974-021-02353-2. J Neuroinflammation. 2021. PMID: 34952603 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

