Quantification of Pulmonary Fibrosis in a Bleomycin Mouse Model Using Automated Histological Image Analysis
- PMID: 28107543
- PMCID: PMC5249201
- DOI: 10.1371/journal.pone.0170561
Quantification of Pulmonary Fibrosis in a Bleomycin Mouse Model Using Automated Histological Image Analysis
Abstract
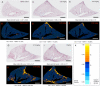
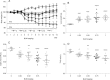
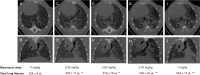
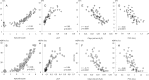
Current literature on pulmonary fibrosis induced in animal models highlights the need of an accurate, reliable and reproducible histological quantitative analysis. One of the major limits of histological scoring concerns the fact that it is observer-dependent and consequently subject to variability, which may preclude comparative studies between different laboratories. To achieve a reliable and observer-independent quantification of lung fibrosis we developed an automated software histological image analysis performed from digital image of entire lung sections. This automated analysis was compared to standard evaluation methods with regard to its validation as an end-point measure of fibrosis. Lung fibrosis was induced in mice by intratracheal administration of bleomycin (BLM) at 0.25, 0.5, 0.75 and 1 mg/kg. A detailed characterization of BLM-induced fibrosis was performed 14 days after BLM administration using lung function testing, micro-computed tomography and Ashcroft scoring analysis. Quantification of fibrosis by automated analysis was assessed based on pulmonary tissue density measured from thousands of micro-tiles processed from digital images of entire lung sections. Prior to analysis, large bronchi and vessels were manually excluded from the original images. Measurement of fibrosis has been expressed by two indexes: the mean pulmonary tissue density and the high pulmonary tissue density frequency. We showed that tissue density indexes gave access to a very accurate and reliable quantification of morphological changes induced by BLM even for the lowest concentration used (0.25 mg/kg). A reconstructed 2D-image of the entire lung section at high resolution (3.6 μm/pixel) has been performed from tissue density values allowing the visualization of their distribution throughout fibrotic and non-fibrotic regions. A significant correlation (p<0.0001) was found between automated analysis and the above standard evaluation methods. This correlation establishes automated analysis as a novel end-point measure of BLM-induced lung fibrosis in mice, which will be very valuable for future preclinical drug explorations.
Conflict of interest statement
The authors declare that their commercial affiliation as given in the funding statement does not alter the adherence to all PLOS ONE policies on sharing data and materials.
Figures

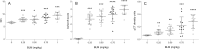

References
-
- Raghu G, Rochwerg B, Zhang Y, Garcia CA, Azuma A, Behr J, et al. (2015) An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline: Treatment of Idiopathic Pulmonary Fibrosis. An Update of the 2011 Clinical Practice Guideline. Am J Respir Crit Care Med 192: 3–19. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

