Hierarchically Ordered Porous and High-Volume Polycaprolactone Microchannel Scaffolds Enhanced Axon Growth in Transected Spinal Cords
- PMID: 28107810
- PMCID: PMC5444512
- DOI: 10.1089/ten.TEA.2016.0378
Hierarchically Ordered Porous and High-Volume Polycaprolactone Microchannel Scaffolds Enhanced Axon Growth in Transected Spinal Cords
Abstract
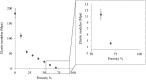
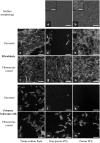
The goal of this work was to design nerve guidance scaffolds with a unique architecture to maximize the open volume available for nerve growth. Polycaprolactone (PCL) was selected as the scaffold material based on its biocompatibility and month-long degradation. Yet, dense PCL does not exhibit suitable properties such as porosity, stiffness, strength, and cell adhesion to function as an effective nerve guidance scaffold. To address these shortcomings, PCL was processed using a modified salt-leaching technique to create uniquely controlled interconnected porosity. By controlling porosity, we demonstrated that the elastic modulus could be controlled between 2.09 and 182.1 MPa. In addition, introducing porosity and/or coating with fibronectin enhanced the PCL cell attachment properties. To produce PCL scaffolds with maximized open volume, porous PCL microtubes were fabricated and translated into scaffolds with 60 volume percent open volume. The scaffolds were tested in transected rat spinal cords. Linear axon growth within both the microtubes as well as the interstitial space between the tubes was observed, demonstrating that the entire open volume of the scaffold was available for nerve growth. Overall, a novel scaffold architecture and fabrication technique are presented. The scaffolds exhibit significantly higher volume than state-of-the-art scaffolds for promising spinal cord nerve repair.
Keywords: controlled porosity; mechanical properties; nerve guidance scaffolds; nerve regeneration; polycaprolactone.
Conflict of interest statement
No competing financial interests exist.
Figures




References
-
- Silva N.A., Sousa N., Reis R.L., and Salgado A.J. From basics to clinical: a comprehensive review on spinal cord injury. Prog Neurobiol 114, 25, 2014 - PubMed
-
- Mahoney M.J., and Anseth K.S. Three-dimensional growth and function of neural tissue in degradable polyethylene glycol hydrogels. Biomaterials 27, 2265, 2006 - PubMed
-
- Xu X.M., Guénard V., Kleitman N., and Bunge M.B. Axonal regeneration into Schwann cell‐seeded guidance channels grafted into transected adult rat spinal cord. J Comp Neurol 351, 145, 1995 - PubMed
-
- McDonald J.W., Liu X.Z., Qu Y., Liu S., Mickey S.K., Turetsky D., Gottlieb D.I., and Choi D.W. Transplanted embryonic stem cells survive, differentiate and promote recovery in injured rat spinal cord. Nat Med 5, 1410, 1999 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

